Abstract
Introduction: MYC and BCL2 overexpression assessed by IHC and rearrangement detected by FISH are important prognostic factor in Diffuse Large B-Cell Lymphoma (DLBCL). Double Hit Lymphoma (DHL) patients have a poor prognosis with conventional therapy and Double expressor Lymphoma (DE) have worse outcome compared with conventional DLBCL although data are controversial. Aimed at a better knowledge of this issue, we performed a retrospective analysis to determine prevalence and outcome of Single Hit Lymphoma (SHL), DHL and DE in patients with de-novo DLBCL treated with Rituximab and CHOP.
Methods: de novo DLBCL treated with R-CHOP between January 2003 and December 2013 were included in the study. BCL2 and BCL6 expression were evaluated by IHC at diagnosis while MYC expression was retrospectively investigated with Tissue Micro Arrays (TMA) technique; cases were considered positive for MYC, BCL2 or BCL6 expression by IHC if >40%, >40% or >25% of cells stained positive, respectively. FISH analysis for MYC and BCL2 rearrangements were performed with dual color break apart probes on TMA. PFS and OS were estimated with Kaplan-Meier method and compared between groups with the Cox model.
We further evaluated in this series the IHC score proposed by Botto et al. (Blood 2014 124:2964) in DLBCL. The score was based on the assessment in IHC of the expression of MYC, BCL2 and BCL6. The three variable contributed with different risk in the multivariate analysis and an IHC sum additive score of 0-5 was calculated proportionally to the coefficient estimated (coefficient [Log hazard ratio] 0.92 for MYC+, 0.73 for BCL2+ and 0.48 for BCL6-), assigning an individual risk of 2 points for MYC or BCL2 positivity and 1 point for BCL6 negativity. Patients were stratified in three different risk groups; Low risk (0-1 point), Intermediate risk (2 points) and High risk (≥3 points).
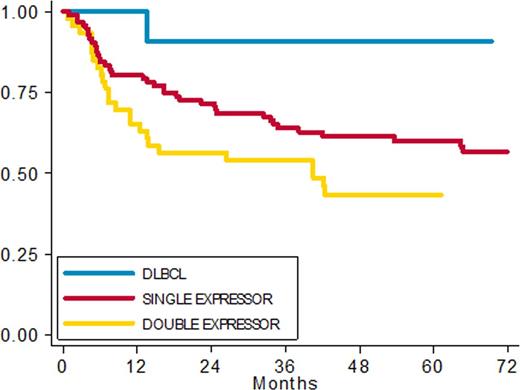
Results: Of a total of299 DLBCL screened, 267 were evaluable for survival analysis; median age was 65 years (range 20-90). 154 patients had complete immunohystochemical data and 101 were fully investigated by IHC and FISH. No significant differences in clinical presentation or in the outcome were seen between patients with or without available histologic tissue for IHC and FISH. Among 154 patients with complete IHC data we found 12 (8%) DLBCL without expression (DLBCL), 96 (62%) Single expressor (SE), 46 DE (30%). With a median follow up of 60 months, 5-year PFS rates were: DLBCL 90%, SE 60% and DE 43% respectively (fig 1) (HR 8.25 (95% CI: 1.12 -60.99) p 0.039); 5ys OS rates were 91%, 68% and 57% respectively (HR 5.72 (95% CI: 0.77 - 42.82) p 0.08).
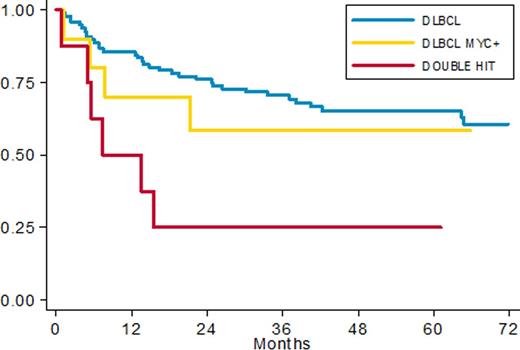
Applying the prognostic model (adjusted for IPI and age) defined in our previous pilot study we recorded 12/154 patients with low risk score, 61/154 with intermediate and 81/154 patients in high risk group. 5y-PFS rates were 91% vs 67% vs 45% (p=0.014) respectively (HR 1.74 (95% CI: 1.12 -2.69) p 0.014). Among 101 patients investigated by FISH we recorded 10 SHL (10%) and 8 DHL (8%). Clinical characteristics were superimposable among DLBCL, SHL and DHL with a prevalence of non GCB phenotype in DE group (56%). Among 101 patients fully investigated for FISH and IHC, 38 patients had MYC overexpression by IHC; 11 of them had also a MYC translocation. We found 3 cases with MYC rearrangement without protein overexpression. With a median follow up of 60 months PFS in DLBCL, SHL and DHL was 65%, 58% and 25% respectively (fig. 2); 5 ys OS was 70%, 77% and 25% respectively. The worse prognosis of DHL was statistically significant with an HR of 3.3 (95% CI: 1.37- 7.94, p 0.008) in terms of PFS and 3.9 (95% CI:1.59- 9.52 p 0.003) in terms of OS.
Conclusion: Our data confirmed that our IHC prognostic score based on MYC, BCL2 and BCL6 expression, is a simple, reproducible and valid prognostic assessment that identify three groups with a different outcome in a large cohort of DLBCL. Moreover these data confirm intermediate prognosis for patients with DE lymphoma and poor prognosis of DHL treated with conventional chemoimmunotherapy.
PFS in patients with complete IHC data
PFS in patients with complete FISH data
Chiappella:Roche: Speakers Bureau; Celgene: Speakers Bureau; Janssen-Cilag: Speakers Bureau; Teva: Speakers Bureau; Pfizer: Speakers Bureau; Amgen: Speakers Bureau. Cavallo:Celgene: Honoraria; Onyx: Honoraria; Janssen-Cilag: Honoraria. Vitolo:Celgene: Honoraria; Gilead: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal