Abstract
Background: Older patients (pts) with acute myeloid leukemia (AML) have a particularly dismal outcome, because of adverse features of AML in the elderly and frailty. The median duration of complete remission (CR) last less than 1 year. The optimal management of older AML pts in daily clinical practice has not been determined. Regular treatment options include best support care, low dose cytarabine (Ara-c) and intensive chemotherapy (anthracycline combined with ara-c). Recently, the DNA methyltransferase inhibitor Azacitidine (AZA) has demonstrated significant activity and favorable tolerability in AML pts also showing a survival advantage.
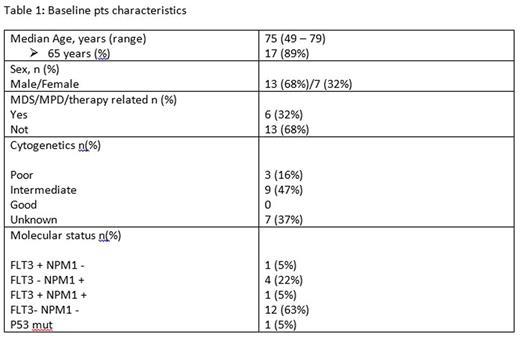
Materials and Methods: Between May 2013 and July 2016, at our institution, 19 pts with a diagnosis of AML (13 males and 7 females) were judged to be ineligible for intensive chemotherapy due to age or comorbidities. They received a 5-day regimen of cytoreductive chemotherapy with ara-c at a dosage of 100 mg\mq\day i.v. continuous infusion. On the sixth day, on termination of Ara-c infusion, all pts had ≤30% bone marrow blasts. Therefore, AZA was administered at a dosage of 75 mg\mq\day subcutaneously for 7 days, continuing the therapy every 28 days. The median age of pts was 75 years (range, 49 to 79 years), with 17 pts (89%) aged over 65 years. Six pts (32%) had poor molecular and cytogenetic risks markers, and six other pts (32%) had either antecedent myelodisplastic/myeloproliferative diseases or therapy related AML. The response to therapy according to the AML IWG criteria was assessed by bone marrow aspiration immediately after Ara-c infusion, after one AZA cycle and every 6 months thereafter. Baseline pts characteristics are summarized in Table 1.
Results: The median number of administered AZA cycles was 6 (range, 1-25 cycles). Fifty eight percent (11/19) of pts received ≥6 AZA clycles. The median overall survival was 6 months (range, 1-26 months). According to AML IWG criteria, 8 pts (42%) achieved CR after Ara-c and a single AZA cycle. Of these, 5 pts (62%) are currently alive in CR, with median duration of response of 7 months (range: 5-12 months), while 3 pts (38%) died after 4, 12, and 22 months after diagnosis. One pt (5%) achieved a partial response (PR) after one AZA cycle, maintaining at present the same response after 3 months of therapy. Other 8 pts (42%) obtained stable disease (SD). Of these, 3 pts (37%) are currently in SD after 2, 8 and 10 months of therapy, while 5 pts (64%) died within a median of 5 months (range: 2 - 18 months) after AML diagnosis. Finally 1 pt (5%) was refractory dying after 2 months of diagnosis, and another pt (5%) died after first AZA cycle for sepsis. Fever and infections were the most common non-hematologic toxic events after Ara-c chemotherapy and first AZA cycle (17/19 pts, 90%). While subsequent AZA cycles were well tolerated.
Conclusion: We suggest that the use of Ara-c-AZA combination is feasible in elderly AML pts. However, the relatively small number of pts studied and short follow up preclude definitive conclusion. The study is still accruing patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal