Abstract
Background:
Aplastic anemia is a rare, serious blood disorder due to bone marrow failure to produce blood cells. Blood transfusions are commonly used to control bleeding and relieve anemia symptoms. In severe patients, transfusions may be required more than once per week. Discrete choice experiments (DCE) can help to understand the trade-offs patients are willing to make to become less reliant on blood transfusion and symptoms associated with the condition and its treatment.
Objective: To elicit patient preferences for attributes associated with treatment of severe aplastic anemia (SAA), including transfusion independence.
Methods:
An online DCE survey was conducted with adults diagnosed with SAA who had insufficient response to immunosuppressive therapy (IST) and experienced transfusion dependence for ≥3 months in the past 2 years. Patients were recruited though announcements in the newsletter of the Aplastic Anemia and Myelodysplastic Syndromes International Foundation and referrals from clinical sites in the U.S. and France. The survey collected information on patient characteristics, treatment, and their views on the impact of achieving transfusion independence on their life. The DCE component asked patients to choose between pairs of hypothetical treatments characterized by a common set of attributes. The attributes were selected based on focus groups conducted with patients and providers: frequency of transfusions (0, 4, and 8 per month), fatigue which interferes with daily activities like work and school (none, moderate, severe), risk of infection (low [ANC>1,000cells/mm3], moderate [500-1,000], high [<500]), and risk of serious bleeding (low [platelet count>50,000], moderate [10,000-50,000], high [<10,000]). A conditional logit model with effects coding was used to estimate part-worth utilities for different attribute levels and assess the relative importance of each attribute. Utility scores associated with different levels of transfusion frequency were also estimated.
Results:
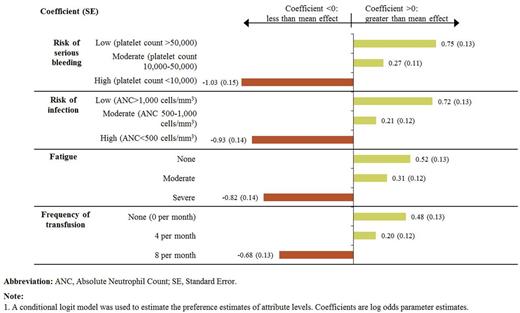
30 patients meeting study criteria completed the survey. Most patients were age ≥40 years (73%), female (70%), and from the U.S. (87%). 50% of patients were diagnosed with SAA within 2 years before the survey; 33% had undergone hematopoietic stem cell transplant; and 37% had received iron chelation therapy. When asked about improvement in quality of life with a new therapy, patients most often noted the following as important: less frequent transfusions, less fatigue, and better emotional well-being (each endorsed by 97% of patients). Patients most often agreed that achieving transfusion independence would result in less burden in terms of time and costs, greater quality of life and life enjoyment, less fatigue, and greater control despite their health condition (each noted by 87% of patients) as well as less need to arrange life around medical appointments (83%). Based on the findings from the DCE, risk of bleeding was the most important factor in patient treatment preferences (0.30 relative importance), followed by risk of infection (0.28), fatigue (0.23), and frequency of transfusions (0.20), based on the range of tested attributed levels. Having more transfusions was associated with lower utility, in particular for increase in frequency of transfusions from 4 to 8 per month (Fig. 1). The mean utility scores estimated by only varying the frequency of transfusion were 0.58 with 0 transfusions per month, 0.57 with 4 transfusions per month, and 0.35 with 8 transfusions per month (Fig. 2).
Conclusion:
Among patients with SAA with insufficient response to IST, the estimated utility was higher with fewer transfusions. While attributes such as risk of bleeding, risk of infection, and fatigue were more important for patient treatment preferences, frequency of transfusions was also important for treatment preferences. Over 80% of patients agreed that achieving transfusion independence would result in less burden on time, less need to arrange life around medical appointments, improved quality of life, less fatigue, and feeling in control despite their health condition.
Preference Estimates for Attribute Levels (Conditional Logit Model),1 N=30
Preference Estimates for Attribute Levels (Conditional Logit Model),1 N=30
Predicted Utility with Different Frequency of Transfusions
Predicted Utility with Different Frequency of Transfusions
Pickard:Analysis Group, Novartis: Consultancy. Ivanova:Novartis: Research Funding; GSK: Research Funding; Teva: Research Funding; Lilly: Research Funding. Huynh:Novartis Pharmaceuticals Corporation, GSK: Research Funding. Totev:Novartis Pharmaceuticals Corporation, AbbVie Inc., Bayer Healthcare Pharmaceuticals LLC, Biogen Idec Inc., Boehringer Ingelheim, Bristol-Myers Squibb Company, GlaxoSmithKline, Janssen Scientific Affairs LLC, Shire Pharmaceuticals Inc.: Research Funding. Graham:Novartis Pharmaceuticals Corporation: Research Funding. Mühlbacher:Analysis Group, Novartis: Consultancy. Roy:Novartis: Employment. Duh:Abbvie: Consultancy; Allergan: Consultancy; Novartis: Research Funding; GSK: Research Funding; Bayer: Research Funding; Janssen: Research Funding; Eisai: Research Funding; Pfizer: Research Funding; Medtronic: Research Funding; Takeda: Research Funding; Novo Nordisk: Research Funding; Sanofi: Research Funding; Ariad: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal