Abstract
The inherited marrow failure syndromes, most importantly Fanconi Anemia (FA) and the Telomere Diseases, are associated not only with marrow failure, but with endocrinopathies, pulmonary fibrosis, cirrhosis and solid organ malignancies. While these disorders classically present in childhood with physical traits and blood count abnormalities, in reality, there is a wide spectrum of clinical findings in these syndromes. Patients may present with solid organ malignancies, pulmonary or liver abnormalities, aplastic anemia (AA) or myelodysplastic syndrome (MDS). Such presentations in adults require a high index of suspicion on behalf of the clinician during the initial stages of diagnosis, as prompt recognition of an inherited marrow failure disorder is imperative to creating an optimal treatment course. Early recognition allows for institution of surveillance programs for solid tumors, routine blood and bone marrow monitoring for the development of AA or MDS, and imparts a certain prognosis. It also allows for screening of additional family members (most important siblings who may be considered as bone marrow donors) and genetic counseling for families affected by these disorders. Treatment is often directed at the underlying bone marrow failure, and as highlighted by the recently published experience at the NIH (Townsley DM et al, NEJM 2016; 374), specific drugs may impact the disease trajectory.
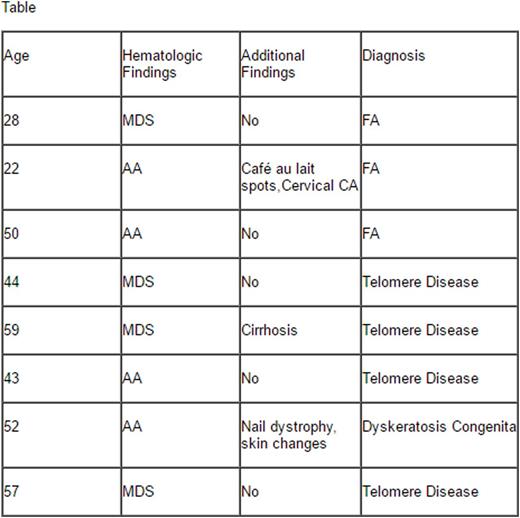
While FA and the other inherited marrow failure syndromes are thought of as primarily diseases of the young, patients can present at older ages. We have therefore established a screening program for patients presenting with MDS under the age of 60, AA patients under the age of 65, and head and neck cancers under the age of 60. Patients presenting with these findings are subject to screening with telomere length testing and blood breakage testing to screen for inherited marrow failure syndromes. With this testing approach, we have identified eight patients with unrecognized inherited bone marrow defects (see Table). Five patients met the criteria for a Telomere Disease, and three patients were diagnosed with FA. Of this subset of patients, only two (20%) had physical characteristics of an inherited bone marrow disorder. In these eight patients, the treatment approach was modulated significantly, including reducing conditioning for BMT, utilizing danazol as first line treatment for AA, and aggressive cancer/endocrinopathy screening.
The importance of recognizing an inheritable syndrome cannot be understated. Treatment options for these patients vary widely compared to the standard approach for acquired MDS and AA. Family members of these patients need to be screened for defects if they are potential bone marrow donors, family members are potentially at increased risk for malignancy and marrow failure, and their offspring are at increased risk of inheritance of the mutated gene. Thus, patients and their family members should be engaged in genetic counselling and encouraged to pursue screening for the inherited marrow failure disorder. Affected individuals should then undergo a comprehensive surveillance program consisting of genetic counseling, and screening for associated endocrine, genitourinary, gastrointestinal, ophthalmologic and hematologic pathology in addition to screening for solid tumors.
Thus, the approach to the congenital/inherited marrow failure syndromes is bimodal. For cases that present in childhood, early recognition can lead to institution of surveillance for malignancy, blood dyscrasia, and marrow failure as well as family counseling via a genetic specialist. Similarly, recognition of delayed presentations is equally paramount, as the adult who presents with MDS, AML, or aplastic anemia is still at increased risk for solid tumors and a more aggressive transformation to a hematologic malignancy. Additionally, identifying a family member with an inheritable condition allows for screening and surveillance of unaffected, or phenotypically silent relatives, with implications ranging from simple counseling and screening, to pre-emptive treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal