Abstract
Introduction
Granulocyte colony-stimulating factor (G-CSF) and its biosimilars remain the only approved therapy for chemotherapy-induced neutropenia to date (Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33(28):3199-212).
Despite the available treatment, patients who experience febrile neutropenia still suffered from a higher risk of mortality (Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116:5555-5563).
Previously we have shown that imidazolyl ethanamide pentandioic acid (IEPA, Myelo001) consistently reduces chemotherapy-induced leukopenia during cyclophosphamide (CP) treatment (Pleimes D, Flechsig S, Meyer M. Effect of IEPA, a Novel Orally Bioavailable Small Molecule, on Chemotherapy-Induced Myelosuppression. Poster presented at American Society of Hematology Annual Meeting and Exposition. December 8, 2014. San Francisco, CA).
Prior non-clinical and clinical studies suggested that the mode of action of Myelo001 is the protection of the hematopoietic bone marrow against the cytotoxic effects of chemotherapy (CTX). A related question is whether Myelo001 also inhibits the effects of CTX on malignant tissue. Here we present the results of the first in vivo study that has investigated whether the effects of CTX on a human breast cancer xenograft in mice remains effective in combination with Myelo001.
Methods
Female athymic nude mice (Crl:NU(NCr)-Foxn1nu) were injected with MDA-MB-231 into the flank. Once the average tumor size reached 100 to 150 mm3, animals were sorted into 6 groups, as shown in Fig. 1, and treatment started. Assessed parameters were: body weight and caliper measurements of tumor size, observation of treatment-related side effects, mortality, and complete blood count (CBC) analysis of blood from mandibular bleeds. The tumor volume was calculated using the following formula:
Results
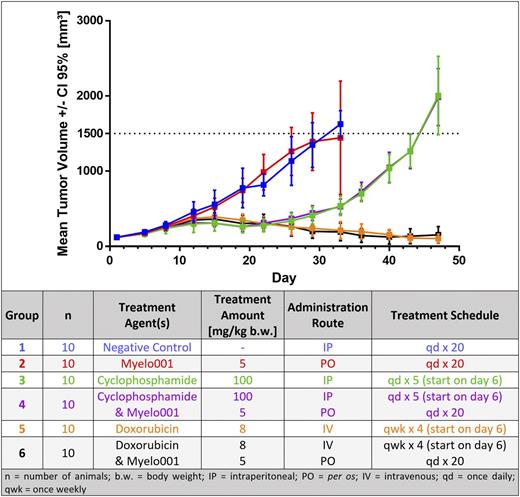
The mean tumor volume of the negative control and Myelo001 group increased exponentially and significantly over the study period (Fig. 1). Both groups treated with CP displayed a tumor growth delay (TGD) until about day 20, after which exponential growth occurred. Treatment with Doxorubicin (Doxo) displayed the same TGD as the CP groups. Furthermore, Doxo was able to reduce the tumor volume consistently throughout the study.
Figure 1: Mean tumor volume for each treatment group, plotted with 95% confidence interval and treatment design of the xenograft study
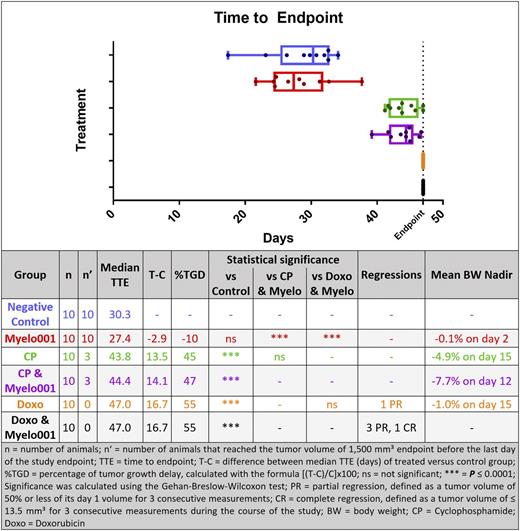
The study endpoint for individual animals was defined as reaching a tumor volume > 1500 mm3 or day 47 of the study, whichever occurred first. No significant difference was observed when comparing the negative control and Myelo001 groups (Fig. 2). Concomitant treatment with Myelo001 together with either CP or Doxo also did not show significant differences compared to the respective group without Myelo001 treatment.
Figure 2: Box-and-whisker plot displaying the time to endpoint for each treatment group and response summary of the presented study
The CBC analysis showed that the median white blood cell and neutrophil counts were significantly smaller for the CP and Doxo groups without Myelo001 on study days 22, 33, and 47 (endpoint) compared to the control group. In combination with Myelo001, the white blood cell count during Doxo treatment displayed a higher trend. The median neutrophil count during treatment with CP and Myelo001 was significantly higher at the endpoint compared to the treatment without Myelo001. (CBC data not shown within the abstract)
Conclusions
This study shows that treatment of human breast cancer xenografts in nude mice with either CP or Doxo was not influenced by concomitant treatment with Myelo001. Thus, the tumor is not protected from CTX when treating leukopenia with Myelo001. The CBC analysis displayed advantages of Myelo001, retaining higher numbers of white blood cells and neutrophils during CTX, but had limitations due to low frequency measurement points and alternating assessment of animals to avoid an influence on the main study objective. In combination with the previous report of the protective properties of Myelo001, this drug is clearly a promising development candidate for chemotherapy-induced myelosuppression.
Brehm:Myelo Therapeutics GmbH: Employment. Donnimath:Myelo Therapeutics GmbH: Employment. Pleimes:Myelo Therapeutics GmbH: Employment, Equity Ownership; CC-Ery GmbH: Consultancy, Honoraria; Bayer: Consultancy, Equity Ownership, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

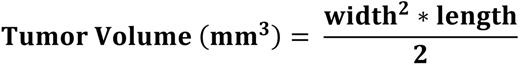
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal