Abstract
Background: Nearly twenty percent of all patients with deep venous thrombosis (DVT) or pulmonary embolism (PE) have an underlying malignancy. Current guidelines recommend low molecular weight heparin (LMWH)-based therapy for venous thromboembolism (VTE) treatment in cancer patients; however, patient considerations including access for treatment and monitoring, co-pay costs and self-administration can be a limitation for its use. Alternative treatments such as direct oral anticoagulants (DOACs) are an attractive alternative to patients and clinicians because of limited monitoring, fixed dosing and limited drug and food interaction. Current guidelines, including those of the American Society of Clinical Oncology and National Comprehensive Cancer Network, do not recommend the use of DOACs for VTE treatment at this time in cancer patients as there is limited data for VTE treatment and secondary prophylaxis with DOAC's.
Methods:We performed a retrospective evaluation of cancer patients at our institution with an active VTE diagnosis who were administered DOACs (rivaroxaban, apixaban and dabigatran) between November 2013 and April 2016. Data collected included patient demographics, diagnosis, and chemotherapy regimen, previous history of VTE, and efficacy and safety during anticoagulation with DOAC's.
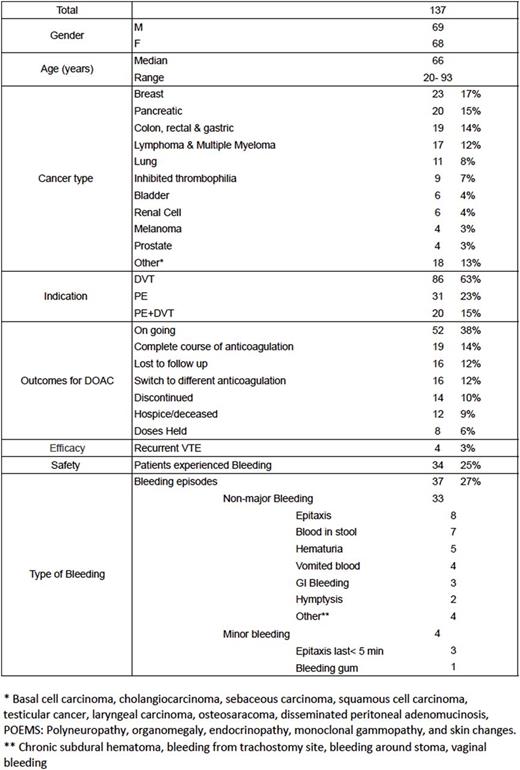
Results: One hundred and thirty-seven patients were included in the study (Table 1), with 112 patients on rivaroxaban, 20 patients on apixaban, and 5 patients on dabigatran. DOACs were administered to treat deep venous thrombosis (DVT) in 86 patients, pulmonary embolism (PE) in 31 patients, and both DVT and PE diagnosis in 20 patients. Only four patients had a secondary clot on therapy during treatment: one patient with pancreatic cancer on apixaban developed recurrent portal vein thrombosis, and three patients with pancreatic cancer, adenocarcinoma of the lung, and Factor V Leiden deficiency on rivaroxaban; 2 patients developed recurrent DVT, and 1 patient developed recurrent PE. Overall, 34/137 (25%) patients experienced a total of 37 bleeding episodes, of which 33/37 were classified as clinically relevant non-major bleeding and 4/37 as minor bleeding. Thirty eight patients had their doses held ,discontinued, discontinued or switched to different anticoagulation therapy; in 11 patients secondary to bleeding, four failed therapy, three experienced intolerance to DOAC, two patients were changed secondary to drug interactions and two patients could not continue therapy secondary to co-pay costs, and two were held prior to surgery. Ten patients had recurrent (>2) bleeding episodes including epistaxis, hematochezia, hematuria, and hematemesis.
Conclusion:In our analysis, DOACs did yield efficacy in cancer patients treated for secondary prophylaxis of VTE with few noted side effects. In our study, DOAC's did not cause fatal or major bleeding. Future prospective studies are warranted for secondary prophylaxis in this setting.
Table 1. Baseline characteristics and outcomes of patients on DOAC's for Secondary DVT prophylaxis
Evaluation of Efficacy and Safety of DOACsin the Treatment of Venous Thromboembolism in Cancer Patients
Evaluation of Efficacy and Safety of DOACsin the Treatment of Venous Thromboembolism in Cancer Patients
McBride:Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal