Abstract
Background
Venous thromboembolism can be classified according to the presence of either environmental or genetic risk factors. Risk factors for thrombosis can include activated protein C resistance, and heritable including deficiencies of antithrombin, protein C or protein S. Factor V Leiden deficiency and prothrombin gene mutations are some of the more common thrombophilias, with a slight increased risk for venous thromboembolism (VTE). Current guidelines suggest the use of low-molecular weight heparins for secondary prophylaxis in patients with VTE. However, there is a lack of data on the use of Direct Oral Anticoagulant (DOACs) in patients with inherited thrombophilia. We evaluated our use of rivaroxaban in patients with thrombophilia disorders treated for secondary DVT prophylaxis.
Method
We performed a retrospective evaluation of patients in our institution with inherited thrombophilia with an active VTE diagnosis who received DOACs for secondary prophylaxis from November 2013 until April 2016. Data collected included patient demographics, inherited thrombophilia mutation, previous history of VTE, prior treatments, and efficacy and safety of anticoagulation with DOACs.
Results
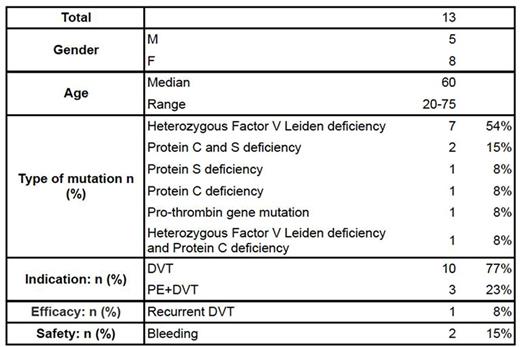
We had 13 patients with inherited thrombophilia mutation and 4 patients diagnosed with concomitant cancer (non-Hodgkin lymphoma, melanoma, and 2 with breast cancer) (Table 1). Out of 13 patients 3 failed warfarin, and one failed fondaparinux prior to switching to a DOAC. Mutation with heterozygous Factor V Leiden deficiency was reported in 7 patients, while mutations with Protein C and/or S deficiency were found in 4 patients. One patient had both Factor V Leiden and Protein C deficiency mutations. The prothrombin gene mutation was identified in one patient. The median of length of therapy was 2 years with 8/13 still on rivaroxaban in April 2016. The shortest treatment duration was 41 days for a patient who failed rivaroxaban with a second clot and was switched to apixaban without subsequent treatment failure. Two patients experienced 4 non-major episodes of gastrointestinal bleeding, nose bleeding and dark stool. One patient developed rash with noted bruising during their rivaroxaban therapy.
Conclusion:
This is the first report on outcomes for secondary DVT prophylaxis with DOACs in patients with underlying thrombophilia mutations. Safety and efficacy of DOACs for secondary VTE prophylaxis yielded favorable results; however, future prospective studies in the setting of thrombophilia are warranted.
McBride:Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal