Abstract
Venous thromboembolic (VTE) disease is associated with significant morbidity and mortality and is one of the leading causes of death in patients diagnosed with a malignancy. The actual reported incidence of VTE in patients with malignancy varies greatly and depends on the type and stage of cancer, type of treatment, and patient-specific factors. This study is designed to assess the efficacy of a novel ultrasound-based technology, Sonic Estimation of Elasticity via Resonance (SEER) Sonorheometry, in predicting thrombosis and identifying high-risk patients.
Treatment naïve patients with a new diagnosis of a solid tumor malignancy were eligible for inclusion in the study. Baseline conventional measures of hemostasis (D-Dimer, fibrinogen, thrombin time, PT/INR, PTT, and platelet count) were measured within the first cycle of chemotherapy and at 6 months for all patients. The HemoSonics' Quantra Hemostasis Analyzer (Quantra) is a point of care device that uses SEER Sonorheometry to measure key parameters of clot formation including clotting time, clot stiffness, and platelet and fibrinogen contributions to clot stiffness. A research use only (RUO) version of the Quantra was utilized to collect data within the first cycle of chemotherapy and at 6 months. Patients were monitored for the development of a venous thromboembolism. In order to determine the feasibility of using this technology/device on a larger scale, our ongoing enrollment goal for this pilot study is 20 patients.
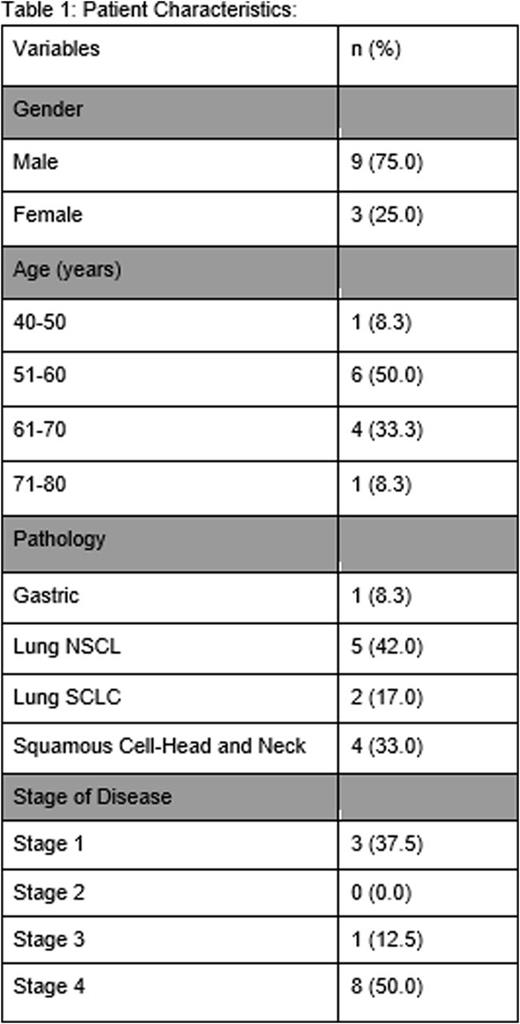
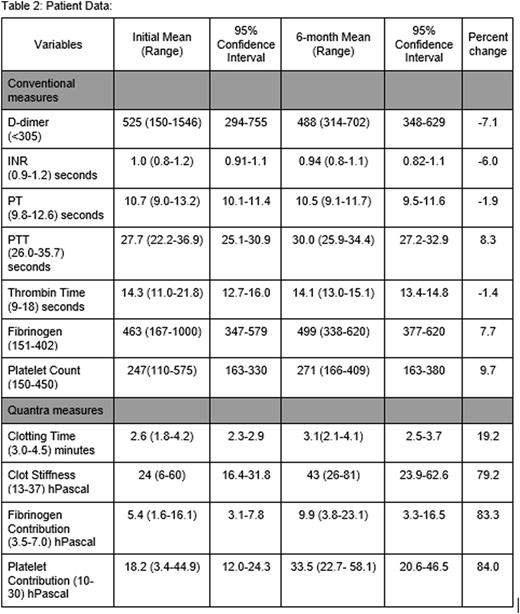
Seven patients with lung cancer (non-small cell lung cancer n=5, small cell lung cancer n=2), 4 patients with squamous cell carcinoma of the head and neck, and 1 patient with gastric adenocarcinoma have been enrolled to date. At the initial and 6-month time points, the mean values for INR, PT, PTT, thrombin time, and platelet count were normal. The mean D-dimer and mean fibrinogen were elevated at both time points. The D-dimer decreased by 7.1 percent over the 6 months while the mean fibrinogen increased by 7.7 percent over the same time period. The mean clotting time increased by 19.2% from 2.6 minutes to 3.1 minutes (95% CI, 2.1-4.1), however it remained within the normal limits of the assay. The mean values for the remaining Quantra parameters increased above the upper limit of normal for the assays over the 6-month time frame. Clot stiffness, fibrinogen and platelet contribution increased by 79.2, 83.3, and 84.0% respectively. Specifically, the mean fibrinogen contribution increased from 5.4 to 9.9 hecto-Pascals (hPa) (95% CI, 3.3-16.5) at 6 months, exceeding the upper limit of normal of 7.0 hPa. The mean platelet contribution to the clot increased from 18.2 to 33.5 hPa (95% CI, 20.6-46.5), exceeding the upper limit of normal of 30 hPa. The mean clot stiffness increased from 24 to 43 hPa over the 6-month interval; the upper limit of normal for the assay is 37 hPa. To date, there have been no documented occurrences of venous thromboembolism in our patient population.
Thromboprophylaxis can be considered for certain high risk patients, however it is not currently recommended as a part of therapy for solid tumors. Additionally, identifying patients at high risk for thrombosis is difficult as no specific biomarkers have been shown to reliably predict VTE risk. SEER Sonorheometry and the Quantra will potentially allow for the ability to identify high risk patients based on parameters that more closely mimic in vivo hemostasis. The number of patients enrolled to date only allows for the observation of potential trends but there is a consistent increase in the clot stiffness as well as platelet and fibrinogen contributions in the patients' hemostatic balance as time progresses. Compared to using D-Dimer for predicting thrombotic risk, which is elevated at both time points, the Quantra parameters tended to be normal at the initial time point and increase over the 6 months to levels that exceeded the upper limit of normal for the assays. While this data suggests a trend toward increasing hypercoagulability with progressive chemotherapy cycles, more data is needed to confirm and understand the significance of these trends and to determine if SEER Sonorheometry and the Quantra can be used to identify those patients at highest risk for thrombosis.
Ferrante:Hemosonics: Employment. Viola:Hemosonics: Employment, Other: Shareholder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal