Abstract
Background: Factor VIII activity (FVIII:C) level is important in the diagnosis and classification of the severity of hemophilia A and can be measured by three methods (one- stage clotting based, two- stage clotting based and chromogenic). About one third of mild / moderate hemophilia A patients show considerable discrepancy in the results of FVIII:C assayed by the above mentioned methods. This group of patients are called "discrepant hemophilia A".
Aims: To determine the prevalence of discrepancy in the results of FVIII:C assays by one- stage and chromogenic methods in Iranian patients with non-severe hemophilia A and the importance of this discrepancy in change of classification of disease severity. We also studied the relationship between the bleeding tendency of these patients with the level of FVIII:C for correct prediction of clinical behavior of the disease.
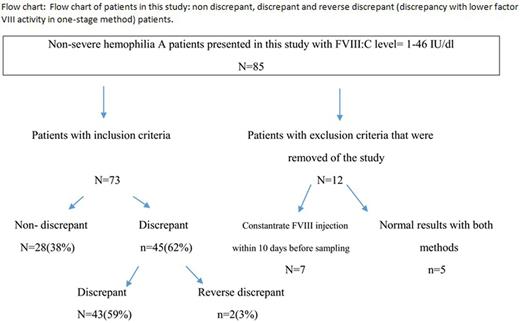
Methods: FVIII:C level was measured using one- stage (FVIII:C1) and chromogenic (FVIII:CR) assays in 78 individuals with mild, moderate or carrier for hemophilia A. Exclusion criteria in our study was considered: receiving FVIII concentrate within 10 days before sampling or having normal results with both methods. Discrepancy was defined as a two- fold or greater difference between the results of two assays. aPTT and mixed-aPTT assays were also performed in all cases. The severity of bleeding symptoms was evaluated using three bleeding assessment tools (BATs): ISTH/SSC BAT, Condensed MCMDM-1VWD bleeding questionary and Vicenza bleeding questionary for the diagnosis of type 1 von willebrand disease.
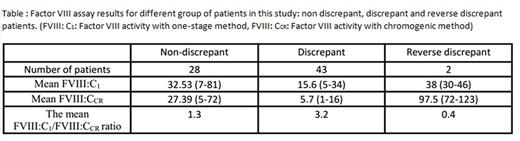
Results: In our study the FVIII:C level was normal with both one-stage and chromogenic methods in 5 patients so they were removed from the data. In the remaining 73 cases, assay discrepancy observed in 45 (62%) patients [43 cases with the lower activity in chromogenic assay (standard discrepancy) and two with the lower activity in the one- stage assay (reverse discrepancy)] ( see the Table and Flow-chart below). Classification of hemophilia A severity changed in 25 (34%) patients (20 patients changed from mild to moderate, 4 cases from mild to normal and 1 patient from normal to mild) based on the results of chromogenic assay. FVIII:C was normal in one patient with one- stage assay but chromogenic assay revealed mild deficiency. As a screening test aPTT did not prolong in 13 (17.8%) of our patients. The relation of ISTH bleeding phenotype with the results of FVIII:C assay by both methods were
statistically meaningful; but not about "Vicenza bleeding questionary" and "Condensed MCMDM-1VWD" one.
Conclusions: Regarding to the high prevalence of assay discrepancy in Iranian patients with non- severe hemophilia A (62%) and changing the classification of the severity of disease in 34% of cases by chromogenic assay, it is recommended that measurement of FVIII:C by both methods should be mandatory in reference coagulation labs in Iran for definitive exclusion of mild hemophilia A, however it is still not clear that which method is completely compatible with the clinical phenotype. Moreover, due to presence of a meaningful relation between ISTH BAT and FVIII:C levels, use of this BAT in hemophilia patients can help to improve the diagnosis accuracy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal