Abstract
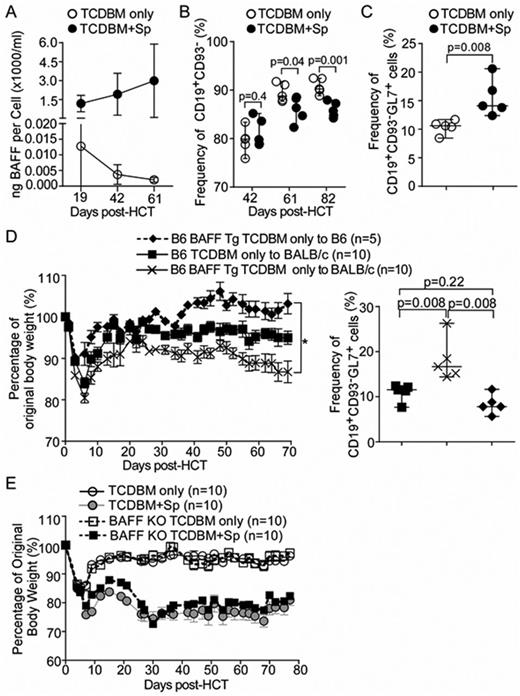
Increased B cell-activating factor (BAFF) and aberrant B cell survival and activation are associated with chronic graft versus host disease (cGVHD) in patients. Whether excessive BAFF production has a pathologic role in the development of cGVHD after allogeneic hematopoietic stem cell transplantation (allo-HCT) remains unknown. Herein, we address this hypothesis by employing BAFF knockout (KO) and BAFF overexpressing (Tg) donor mice in the major MHC mismatched C57BL/6 (B6)-to-BALB/c cGVHD model (Wu et al J Immunol 2013;191:488), except we increased the T cell depleted bone marrow (TCDBM) dose to 1x107 per recipient to improve engraftment. BALB/c recipients receiving TCDBM plus 1x106 splenocytes (Sp) are the disease group, and those receiving TCDBM only are controls. After verifying cGVHD phenotypic manifestations (including longevity, weight loss, eye and lung findings), we examined whether cGVHD mice had the increased BAFF and B cell findings as occur in human cGVHD (Sarantopoulos et al, Blood 2009; 113: 3865). We observed that cGVHD mice had higher BAFF/B cell ratios compared to controls (Fig. 1A, n=10 each). We fully characterized the peripheral B cell compartment in these mice, and found that the relative composition of B cell subsets was significantly altered in diseased mice. CD93 cell surface expression has been used in mice to define B cells that emigrate from BM and circulate through secondary lymphoid organs. In cGVHD mice, we found a significant increase in the relative proportion of CD93+ B cells and a significant decrease in the proportion of CD93- B cells (Fig1B). Also, a significant increase in the frequency of cells positive for the germinal center and activation marker GL7 was found only within CD93- B cell subset of cGVHD mice (Fig 1C). When stimulated via BCR ex vivo, this GL7+ mature B cell subset had significantly increased Syk and BLNK activation, measured by phosphoflow (n=5, p=0.008). We next aimed to determine whether high BAFF alone or high BAFF and alloantigen together lead to altered B cell homeostasis and to the promotion of GL7+ BCR-activated B cells. C57BL/6 (B6) BAFF Tg TCDBM cells were used as donor to afford excessive and persistent BAFF levels after HCT in either syngeneic or BALB/c recipients. After B6 BAFF Tg TCDBM to BALB/c HCT, body weight decreased significantly. By contrast, B6 BAFF Tg TCDBM to B6 syngeneic HCT recipients remained healthy (Fig.1D, left panel,*p=0.004). Notably, the frequency of the CD93-GL7+ B cell subset was increased in BALB/c mice that received B6 BAFF Tg TCDBM only, compared to recipients of syngeneic BAFF Tg or WT TCDBM only (Fig.1D, right panel, p=0.008). Thus, both BAFF and alloantigen are required for B cell activation after HCT. Together, our data also suggest that GL7+ B cells identify aberrantly BCR-activated B cells in murine cGVHD, arising in the setting of BAFF-driven altered B cell homeostasis.
While polymorphisms in either donor or recipient BAFF genes are associated with human GVHD (Clark et al Blood 2011; 118: 1140), the source of excess BAFF after allo-HCT remains unknown. We addressed whether pathologic BAFF was derived from donor or recipient hematopoietic cells using TCDBM from B6 BAFF KO mice vs WT as donor cells for HCT to BALB/c recipients. Consistent with the known requirement of recipient BAFF for recovery of the B cell compartment after syngeneic transplant (Gorelik et al JEM 2003; 198:937), recovery of a donor peripheral B cell pool was not significantly different between groups. Remarkably, plasma BAFF levels were also not different between BALB/c recipients of B6 BAFF KO TCDBM +Sp vs recipients of WT TCDBM +Sp, suggesting that radioresistant recipient cells produced BAFF in cGVHD. Chronic GVHD-associated weight loss was also not different between groups (Fig.1E, representative of 3 separate experiments). We found that BAFF production in the engrafted KO recipient BM was uniformly low. Thus, we have now determined that non-BM, radioresistant recipient cells are the principal source of soluble pathologic BAFF in murine cGVHD. Further characterization of BAFF-producing cells is underway in order to identify therapeutic targets.
In summary, we have now demonstrated that recipient-derived BAFF has a mechanistic role in aberrant activation of B cells in cGVHD. Our findings will lead to the development of anti-BAFF and BCR targeting agents for patients.
This work was supported the National Institutes of Health (NHLBI R01 HL 129061-01).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal