Abstract
Pharmacokinetic (PK) parameters are important surrogate indicators for hemostatic efficacy replacement therapy in the treatment of haemophilia patients. Nonacog gamma (Rixubis) is a recombinant factor IX (rFIX) concentrate that is manufactured using two viral inactivation steps (solvent/detergent treatment and nanofiltration) and without any materials of animal origin. Clinical trials of nonacog gamma included a comprehensive evaluation of PK in hemophilia B patients across multiple age groups and a direct comparison to determine equivalence with another licensed, commercially-available factor IX product. The objective of this evaluation was to assess the factor IX activity across clinical development with nonacog gamma.
PK parameters for nonacog gamma were evaluated in a non-bleeding state in previously-treated patients with severe (FIX level < 1%) or moderately severe (FIX level 1-2%) hemophilia B in three prospective clinical trials (a pivotal trial in adults,1 a pediatric trial in children aged 2 to 12 years,2 and a third trial in patients undergoing surgical procedures3).
Factor IX activity was determined using a one-stage clotting assay. In the pivotal trial, PK equivalence with a comparator recombinant factor IX product (nonacog alfa) was determined by the ratio of AUC0-72 h (area under the plasma concentration versus time curve from 0 to 72 hours post-infusion), per dose with a type I error of 5% for the two-sided 90% confidence interval (equivalence margin: 80% to 125%).
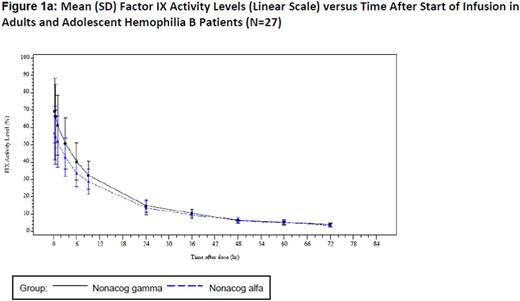
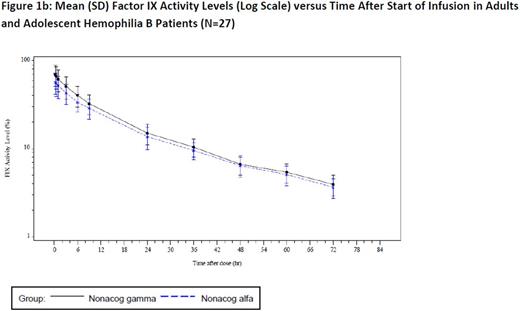
Windyga et al. (2014) established bioequivalence of nonacog gamma with a comparator recombinant factor IX in the pivotal trial (N=27), as the 90% CI for the ratio of the AUC0-72 h per dose ranged from 103% to 109%.1 A plot of mean factor IX activity levels versus time after start of infusion on a linear scale and log scale (figure 1a and 1b) depicts the equivalence throughout the 72-hour follow-up period. A similar pattern can be observed in the linear and log regression for mean factor IX activity in pediatric patients (N=23). These results are further supported by consistent PK parameters determined prior to surgical intervention in the surgery trial (N=12).3 Two important PK parameters determined in each study were as follows: mean [STD] T ½ 26.70h (9.55), incremental recovery (IR) 0.87 (0.22) for nonacog gamma and 27.87h (9.22), IR 0.76 (0.20) for the comparator rFIX in the pivotal trial, 25.31h (3.130), IR 0.67 (0.16) in the pediatric trial, and 23.60h (3.60), IR 1.06 (0.35) in the surgery trial.
Conclusion
Factor IX activity of nonacog gamma throughout its clinical development support previous finding of its equivalence with another licensed recombinant factor IX concentrate in patients with severe to moderately severe hemophilia B.
References
1. Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicentre phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level ≤2%) haemophilia B. Haemophilia. 2014 Jan;20(1):15-24.
2. Urasinski T, Stasyshyn O, Andreeva T, et al. Recombinant factor IX (BAX326) in previously treated paediatric patients with haemophilia B: a prospective clinical trial. Haemophilia. 2015 Mar;21(2):196-203.
3. Windyga J, Lissitchkov T, Stasyshyn O, et al. Efficacy and safety of a recombinant factor IX (Bax326) in previously treated patients with severe or moderately severe haemophilia B undergoing surgical or other invasive procedures: a prospective, open-label, uncontrolled, multicentre, phase III study. Haemophilia. 2014 Sep;20(5):651-8.
Windyga:Baxalta, now part of Shire: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Investigator Clinical Studies, Patents & Royalties, Research Funding, Speakers Bureau; Nordisk: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Sanofi: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Biogen: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Octapharma: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Alexion: Other: Speaker's honorarium; Aspen: Consultancy, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau. Chapman:Baxalta (Now part of Shire): Employment, Equity Ownership. Tangada:Baxalta US Inc., now part of Shire: Employment, Equity Ownership. Chatterjee:Baxalta (Now part of Shire): Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal