Abstract
INTRODUCTION: FuseNGo is a dual-chamber syringe for administering coagulation factor concentrate that was developed to reduce the number of steps and components necessary to reconstitute the drug product. This study aimed to assess patients' perception and preference for Haemophilia A (HA) using a recently developed questionnaire (HaemoPREF). The study also aimed to build on existing evidence for the construct validity of the HaemoPREF by evaluating the relationship between patient perception and preference for HA treatment, as assessed by the HaemoPREF, and associated factors including treatment satisfaction, quality of life (QoL), adherence, work productivity and activity impairment.
METHODS: A non-interventional, cross-sectional, questionnaire completion study was conducted in Spain (n=31), Germany (n=10) and Italy (n=48). Adult HA patients were required to have used ReFacto AF with FuseNGo for at least 40 infusions. Spanish and German patients completed a web-based survey; Italian patients' data was collected on paper as part of a nested sub-study in an ongoing real-world study. Patients completed: HaemoPREF; Treatment Satisfaction Questionnaire for Medication (TSQM); VeritasPRO; Hemophilia Well-being Index (HWBI) and Work Productivity and Activity Impairment: Haemophilia Specific (WPAI + CIQ: HS). Correlational analyses, univariate and multivariate regression analyses aimed to examine the relationship between HaemoPREF and these instruments.
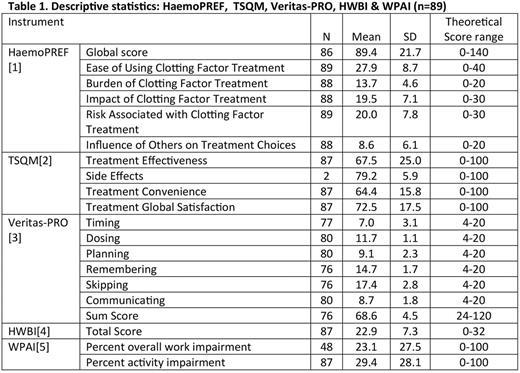
RESULTS: Higher scores on the HaemoPREF indicate greater satisfaction with, or importance of, a concept. Scores are provided as a fraction of the theoretical range. The subscales with the greatest mean scores are 'Ease of Using Clotting Factor Treatment' (27.9/40); and 'Burden of Clotting Factor Treatment' (13.7/20) indicating high levels of satisfaction. The subscale with the lowest mean score is 'Influence of Others on Treatment Choices' (8.6/20). Scores on the TSQM indicated overall satisfaction with treatment, in particular the 'Treatment Global Satisfaction' subscale was high on average (72.5/100). The mean VeritasPRO global score indicated that the sample was moderately adherent overall. The mean score on the HWBI was 22.9/24, indicating patients do not consider their QoL to be negatively affected. On the WPAI the 'Overall Work Impairment' mean score was 23.1% while the 'Activity Impairment' mean score was 29.4% indicating low levels of work and activity impairment (Table 1).
Spearman's correlations indicated the HaemoPREF global score had essentially a moderate relationship with the TSQM Treatment Global Satisfaction subscale (r=0.48), HWBI total score (r=0.41) and WPAI Activity Impairment score (r=0.39). Moderate correlations (≥0.40) were also noted between some of the HaemoPREF subscales. Some subscales on the TSQM, HWBI and WPAI. Correlations were low between the HaemoPREF and VeritasPRO (r=0.24) and the HaemoPREF and the WPAI Overall Work Impairment score (r=0.31) indicating the HaemoPREF is addressing something conceptually distinct to these measures.
Multivariate regression on the pooled sample for the HaemoPREF global score (n=87) found the following variables were significant (p≤0.05) predictors of HaemoPREF global score: TSQM 'Treatment Convenience' (regression coefficient estimate: 0.5), VeritasPRO 'Timing' (regression coefficient estimate: 2.8), VeritasPRO 'Remembering' (regression coefficient estimate: 3.1), HWBI Total Score (regression coefficient estimate: 1.0) and site (Germany/Spain vs Italy, regression coefficient estimate: 13.0).
CONCLUSIONS: These findings suggest that patients using ReFacto AF with FuseNGo are satisfied with and adherent to their treatment, and do not consider their QoL to be negatively affected. There is a moderate association between patient perception and preference for HA treatment, treatment satisfaction, adherence and QoL. This suggests while the HaemoPREF is related to, and assesses these components, it also measures distinct and important concepts not assessed by the other questionnaires completed by patients in the study. Thus, the HaemoPREF appears to fill a gap in the tools currently available to assess patient treatment experience in HA. Additional research in larger samples across multiple sites could further evaluate the relationship between the factors of interest.
Schulz:Pfizer Inc: Employment. Gordo:Pfizer Inc: Employment. Tolley:Adelphi Values: Other: full-time employee of Adelphi Values, who worked on this study as paid contractors to Pfizer Inc.. Staunton:Adelphi Values: Other: full-time employee of Adelphi Values, who worked on this study as paid contractors to Pfizer Inc.. Brohan:Adelphi Values: Other: full-time employee of Adelphi Values, who worked on this study as paid contractors to Pfizer Inc.. Spurden:Pfizer Inc: Employment. Cicchetti:Pfizer Inc: Other: paid consultant through Execupharm Inc. by Pfizer Inc.. Cappelleri:Pfizer Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal