Abstract
Introduction: Acquired hemophilia A (AHA) is a rare (1.5 per million/year) autoimmune disorder due to formation of spontaneous antibodies against factor VIII (FVIII) with an estimated mortality of 8-22%. First-line hemostatic agents to control a bleed include activated prothrombin complex concentrate (aPCC) and recombinant activated factor VII (rVIIa) whereas immunosuppressive therapy to eradicate the inhibitor include prednisone, cyclophosphamide, and rituximab. We present data on 12 AHA patients from our institution.
Methods: We conducted a retrospective review of patients with AHA from January 1, 2010 to July 1, 2016.
Results: Eight women and 4 men with a median age of 68 years (range 29-88 years) were included in the study. AHA was idiopathic in 7 patients, others being associated with HIV/Hepatitis C (1), Hepatitis C only (1), lymphoma (1) pregnancy (1), and pregnancy/systemic lupus erythematosus (SLE) (1).
Of the 12 patients, 11 were admitted at least once with a bleeding episode. In total, there were 38 bleeding episodes, with 15 being characterized as severe (39%). The most common site of bleeding was deep muscle (50%), and 71% of bleeding episodes were considered spontaneous. The median FVIII activity at admission was <1% (range <1%-5%) with a median FVIII inhibitor titer of 102.4 BU/ml (range 7.4 - 5734.4 BU/ml).
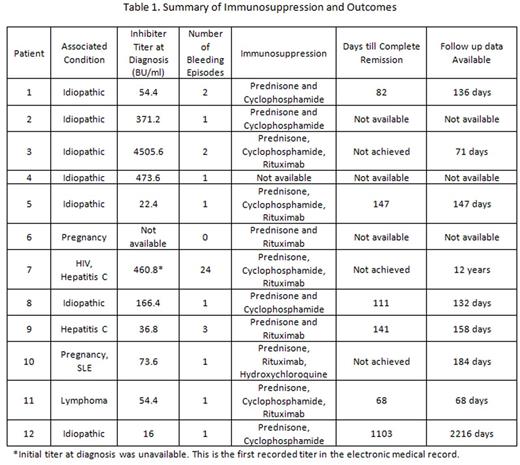
Table 1 summarizes the number of bleeding episodes and immunosuppression for each patient. Patient 7 experienced 24 bleeding episodes with 23 requiring both aPCC (median 75 u/kg, range 50-80 u/kg) and rVIIa (median 50 mcg/kg, range 50-90 mcg/kg) alternating every 6 hours. This patient had breakthrough bleeds on either aPCC or rFVIIa but responded to the alternating regimen. Patient 11 developed severe bleeding in his upper extremities, necessitating bilateral upper extremity amputation. He was initially treated with only aPCC (100u/kg every 12 hours for 11 doses total); however, concern for disseminated intravascular coagulation prompted aPCC discontinuation. He received a total 121 doses of rVIIa (90mcg/kg every 2-4 hours) during his hospital stay. Patient 2 required two doses of rVIIa (90 mcg/kg every 12 hours) after having continued bleeding despite treatment with 11 doses of aPCC (50u/kg every 12 hours). Ten bleeding episodes were treated with only aPCC with a median 8 doses (range 2-16 doses), while 1 bleeding episode was controlled with only rVIIa. One bleeding episode did not require treatment. The median dosage of aPCC (with or without rVIIa) was 75u/kg (range 50-100 u/kg) given every 12 hours (range 8-24 hours) for 8 doses (range 1 -16 doses). The median dosage of rVIIa when used in conjunction with aPCC was 50mcg/kg (range 50-100 mcg/kg) given every 12 hours (range 4-24 hours) for 6 doses (range 1-13 doses). No patient experienced a thromboembolic event.
Follow-up data was available for 9 patients (Table 1). Six patients achieved complete remission (CR) at a median 126 days (range 68-1103 days). Patient 12 was initially treated with 1mg/kg prednisone; however, upon tapering, her FVIII activity shortened from 114% to 36%. Cyclophosphamide (2mg/kg) was added with eventual inhibitor eradication. Patients 3 and 7 failed to attain CR and were treated with prednisone, cyclophosphamide, and rituximab. They continued to have a FVIII activity <1% at 71 days and 12 years follow-up respectively. Patient 11 had pregnancy and SLE associated AHA. She was treated with 1mg/kg prednisone, 1 dose of rituximab, and hydroxychloroquine for her concurrent SLE. Her inhibitor titer was undetectable at 89 days, but her FVIII activity remained at 36% (blood type O) after 184 days.
Conclusion: CR was achieved in 6 of 9 patients with follow-up data. There were no deaths related to AHA. A combination of aPCC and rVIIa, alternating every 6 hours, can safely be used to treat severe bleeding, reducing drug and administrative costs.
Sarode:CSL Behring: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal