Abstract
Introduction: Plasminogen activator inhibitor-1 (PAI-1) is an important component of the senescence-associated secretome that contributes directly to cellular senescence. Telomeres, the nucleotide repeat structures located at the ends of chromosomes, shorten progressively with each round of cellular replication and telomere attrition is associated with cellular senescence. In murine models, genetic or pharmacologic inhibition of PAI-1 results in preservation of telomere length. Identification of a unique Amish kindred that harbors a loss-of-function mutation in SERPINE1 (c.699_700dupTA), which encodes PAI-1, provides a novel opportunity to assess the effects of lifelong PAI-1 deficiency on leukocyte telomere length (LTL) in humans.
Methods: We conducted an observational study in the Old Order Amish, a founder population who originally settled in Berne, Indiana characterized by a homogeneous diet and lifestyle, and included participants aged 18 years and older. All study participants were genotyped for the c.699_700dupTA frameshift mutation in SERPINE1. For the primary analysis, we excluded participants who were homozygous for the null SERPINE1 mutation (n=7) due to their young age (18-34 years old) and small sample size. Relative LTL was quantified from peripheral blood using Southern blot of the terminal restriction fragments. We tested the association of SERPINE1 mutation status with LTL, after adjustment for age, sex, and relatedness in SOLAR.
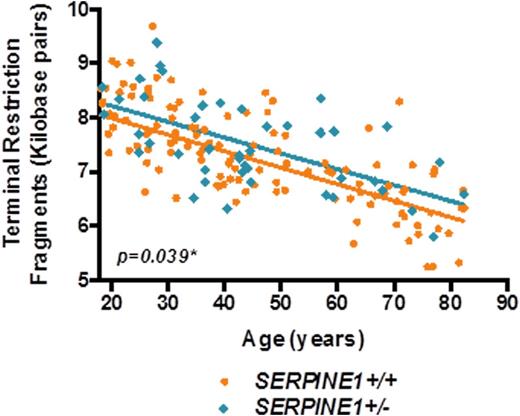
Results: A total of 170 participants were enrolled with mean age 46±19 years. Forty-three participants were identified as carriers of the null SERPINE1 mutation with an overall minor (null) allele frequency of 16% in the study population. SERPINE1 carriers had a significantly greater LTL compared with noncarriers, after adjustment for age, sex, and family structure (p=0.039; FIGURE). Every 1-year increase in age of study participant was associated with a 30 base pair decrease in LTL (p<0.0001).
Conclusions: Heterozygosity for the null SERPINE1 gene, encoding a senescence-associated protein, PAI-1, is associated with longer LTL in a rare loss-of-function cohort. In addition, LTL is strongly and inversely correlated with chronologic age and supports the hypothesis that PAI-1 may contribute to cellular and organismal aging as reflected by LTL.
Mean telomere restriction fragments (kilobase pairs) as a function of age and genotype
Mean telomere restriction fragments (kilobase pairs) as a function of age and genotype
Shapiro:CSL Behring: Other: Clinical research protocols; Kedrion Biopharma: Consultancy; Daiichi Sankyo: Other: Clinical research protocols; OPKO: Other: Clinical research protocols; National Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Other: Clinical research protocols; Novo Nordisk Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; Baxter/Baxalta/Shire: Membership on an entity's Board of Directors or advisory committees, Other: Clinical research protocols; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Other: Clinical research protocols; Biogen: Membership on an entity's Board of Directors or advisory committees, Other: Clinical research protocols; Genentech: Membership on an entity's Board of Directors or advisory committees; American Thrombosis and Hemostasis Network: Other: Medical Director; ProMetic Life Sciences: Consultancy; Bayer healthcare Pharmaceuticals: Other: International network on pediatric hemophilia; Novartis: Other: Clinical research protocols; Selexys: Other: Clinical research protocols; Octopharma: Other: Clinical research protocols.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal