Abstract
Background.
Cancer patients often display procoagulant activity and are at a risk for developing venous thromboembolism (VTE). Multiple mechanisms may explain this procoagulant state, such as tumor cell expression of tissue factor (TF), host tissue response to tumor formation and also contributions from comorbid factors and treatment. We previously observed elevated levels of traditional biomarkers of coagulation (such as D-dimer, CRP, etc.) in patients with advanced cancer on therapy enrolled in a randomized trial comparing rosuvastatin versus placebo. In this study, we evaluated the frequency and concentration of endogenous plasma TF, Factor (F)XIa and FIXa in the cancer patient population analyzed for traditional coagulation biomarkers. The choice of new analytes was based on previous studies in which these 3 proteins were detected and quantitated in patients with cardiovascular diseases, inflammation, and trauma - often present over the course of several days to even weeks. By our knowledge, there are no published studies of the presence of FXIa and/or FIXa in cancer patients.
Methods.
Thirty-seven adult cancer patients receiving systemic therapy were originally enrolled in a randomized crossover trial comparing effects of rosuvastatin versus placebo on biomarkers of VTE. Inclusion criteria included locally-advanced or metastatic cancer, estimated survival time of greater than 6 months, and anticipated duration of therapy of at least 3 months. Patients on antithrombotic or statin therapy were excluded. Blood was collected on up to 4 occasions; at the start and end of each of 2 treatment periods corresponding to 2 cycles of systemic therapy with a 3-4 week washout period. Citrate plasma was prepared, frozen and stored at -80C. The assay, performed in previously unthawed and contact-pathway inhibited plasma, is based on a response of the lag phase of thrombin generation to corresponding monoclonal inhibitory antibodies (αFXIa-2, αFIXa-91 and αTF-5; all at 0.1 mg/mL). Concentrations of TF, FXIa and FIXa were calculated from corresponding calibration curves built by titrating purified proteins into multi-donor pooled plasma from healthy individuals.
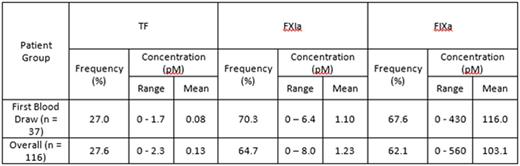
Results.
A total of 116 blood samples were collected (not all patients were able to complete their regime) and analyzed for endogenous levels of TF, FXIa and FIXa (see table). Overall, rosuvastatin treatment did not have any significant impact on the levels and frequency of these 3 proteins. For the samples obtained from each patient's first blood draw (n = 37) there was a weak correlation between FXIa and FIXa levels (R = 0.37, P = 0.02) and a moderate correlation between FXIa and TF levels (R = 0.57, P < 0.001), while no correlation between FIXa and TF was observed (R = 0.02, P = 0.91).
Conclusions.
1) A portion of patients with advanced cancer had active TF in their plasma while the majority, for the first time reported, had active FXIa and FIXa; 2) No pronounced differences were observed between the frequency and concentrations of these proteins at baseline versus later time-points; 3) There was a weak correlation between FXIa and FIXa and a moderate correlation between FXIa and TF, suggesting the TF-pathway is driving the majority of FXIa generation, although contribution from the contact pathway cannot be excluded; 4) Rosuvastatin treatment did not lead to any significant changes in the levels of TF, FXIa and/or FIXa.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal