Abstract
This study investigated the safety and efficacy of IV-Globulin SN, a 10% intravenous immunoglobulin (IVIg), in patients with severe ITP (platelet count ¡Â20 x 109/L). Among 81 eligible patients, 31 patients were newly diagnosed, 7 patients had persistent ITP, and 43 patients had chronic ITP. Five patients had received splenectomy. Patients received IV-Globulin SN 1 g/kg/day on two consecutive days; infusion rate was initially 1 mg/kg/minute and then doubled every 30 minutes to a maximum of 8 mg/kg/minute.
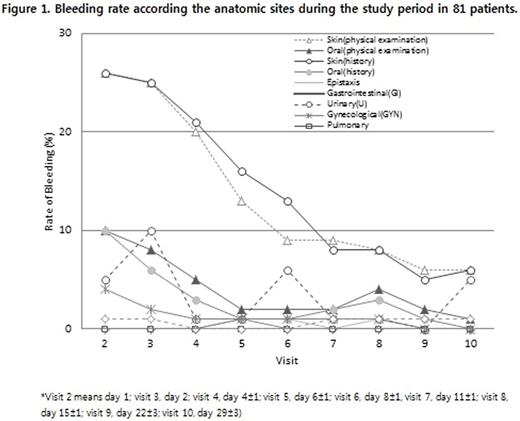
Fifty-eight patients (72%) attained the primary efficacy endpoint of clinical response (platelet count ¡Ã 50 x 109/L within 7 days). Their median time to response was 2 days and the median duration of response was 10 days (range from 1 to 28 days). Complete response (platelet count ¡Ã 100 x 109/L over 7 days) was obtained in 14 patients (17%). Response rates were not significantly different when compared the patients with newly diagnosed, persistent or chronic ITP; previous treatment with immunosuppressant or not; splenectomized or not. IV-Globulin SN 10% was well tolerated and the frequency of mucocutaneous bleeding was decreased during the study period (figure 1). There was no unexpected adverse event. The mean half-life and Cmax of study drug were 28.9 days and 34.6 g/L in 25 tested patients. There were no unexpected adverse events. In conclusion, IV-Globulin SN was efficacious in adult ITP patients regardless of their disease status as well as safe given that the resolution of bleeding and minimal infusion-related adverse events. (NCT02063789)
Bleeding rate according the anatomic sites during the study period in 81 patients.
*Visit 2 means day 1; visit 3, day 2; visit 4, day 4¡¾1; visit 5, day 6¡¾1; visit 6, day 8¡¾1, visit 7, day 11¡¾1; visit 8, day 15¡¾1; visit 9, day 22¡¾3; visit 10, day 29¡¾3)
Bleeding rate according the anatomic sites during the study period in 81 patients.
*Visit 2 means day 1; visit 3, day 2; visit 4, day 4¡¾1; visit 5, day 6¡¾1; visit 6, day 8¡¾1, visit 7, day 11¡¾1; visit 8, day 15¡¾1; visit 9, day 22¡¾3; visit 10, day 29¡¾3)
Kim:Green Cross Corp.: Employment. Lee:Green Cross Corp.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal