Abstract
Background: ITP is a rare autoimmune hematologic disorder characterized by isolated thrombocytopenia caused by antibody-dependent platelet destruction and impaired platelet production. Several therapeutic options (eg glucocorticoids, intravenous immunoglobulin and thrombopoietin receptor agonists) are available to treat patients with ITP, although inadequate efficacy, side effects and/or cost can make them undesirable. PRTX-100 is a highly purified form of Staphylococcal protein A (SpA), which binds to human B-lymphocytes and monocytes, and modulates immune processes. Preclinical data indicate that PRTX-100 may have the potential to treat ITP by reducing immune-mediated destruction of platelets. Here we present safety/efficacy data from the scheduled analysis for dose escalation from the initial dose cohorts of patients with ITP enrolled in two Phase 1/2 open-label studies, PRTX-100-202 and PRTX-100-203.
Methods: Adult patients with persistent/chronic ITP and either a platelet count <30,000/µL (if not receiving any ITP therapy) or a platelet count of <50,000/µL (if receiving a constant dose of permitted ITP treatment) were eligible for inclusion. Study 202 requires prior treatment with a thrombopoietin receptor agonist (TPO-RA) plus one additional standard ITP treatment, while Study 203 only requires prior treatment with one standard ITP treatment. In both studies, PRTX-100 was administered via a 30-min infusion (60 min if total dose >500 µg) on Days 1, 8, 15 and 22 in a standard 3 + 3 dose-escalation design. Starting dose was 1 µg/kg (Study 202) or 3 µg/kg (Study 203) with subsequent dose increases planned up to a maximum of 24 µg/kg. Platelet counts were monitored on Days 1, 3, 8, 15, 22, 29, 36, 43, 50, 78 and 106. Safety analyses included consideration of adverse events (AEs), serious AEs, infusion reactions, clinical laboratory tests, vital signs, physical findings and electrocardiograms. The key efficacy endpoint was platelet response defined as: a platelet count ≥30,000/µL and at least a doubling of baseline platelet count in patients with a baseline platelet count <30,000/µL; or, in patients receiving permitted treatments for ITP with a baseline platelet count ≥30,000/µL and <50,000/µL, an increase in platelet count to ≥50,000/µL and at least a doubling of baseline platelet count or an increase to >100,000/µL.
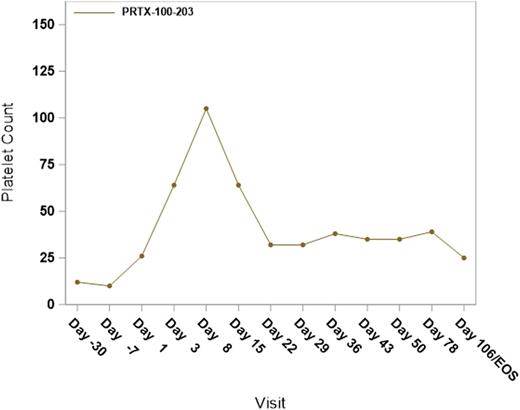
Results:Data are available from 6 patients (Study 202 n=3; Study 203 n=3) enrolled in the lowest-dose cohorts of the PRTX-100 ITP studies. There were 3 women and 3 men who were all Caucasian and ranged in age from 49 to 78 years; four patients had a splenectomy. Concomitant ITP medications were prednisone (n=4), dapsone (n=1), and TPO-RAs (n=2). Two patients in Study 202 received four doses of PRTX-100 (1 µg/kg); three patients in Study 203 received four doses of PRTX-100 (3 µg/kg). One patient receiving a dose of 1 µg/kg (Study 202) experienced a grade 3 vasculitis allergic reaction and discontinued from the study after 2 doses. This patient had a history of milder allergic reactions to other therapies. No additional serious or grade >3 AEs or immediately reportable events have been reported for any patient. Three grade 3 laboratory abnormalities have been reported: one patient (1 µg/kg; Study 202) experienced decreased hemoglobin and hyperkalemia, and one patient (1 µg/kg; Study 202) experienced high white blood cell count. One grade 1 infusion reaction occurred (itching rash at the infusion site in one patient receiving 3 µg/kg [Study 203]). No other infusion reactions were reported. Baseline platelet counts ranged from 6,000 - 30,000/µL. One patient in Study 203 (3 µg/kg) had a platelet response with a peak platelet count of 105,000/µL following the initiation of treatment. Platelet count elevation was observed as early as Day 3 and remained elevated for 2-3 weeks following the initiation of treatment (Figure 1).
Conclusions:Data from initial cohorts in two dose escalation trials of patients treated with PRTX-100 at a dose of 1 µg/kg or 3 µg/kg have demonstrated an acceptable safety profile to support continued enrollment into higher-dose cohorts in both trials. A platelet response was observed in one patient treated at the lowest dose in one of the trials. Enrollment into higher-dose cohorts is ongoing and updated data from patients treated in those cohorts will be included in any presentation.
Bussel:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Prophylix Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Symphogen: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Research Funding; Momenta Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Shionogi: Membership on an entity's Board of Directors or advisory committees; Immunomedics: Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau; UpToDate: Patents & Royalties; Sysmex: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; BiologicTx: Research Funding. Kuter:Syntimmune: Consultancy; MedImmune: Consultancy; Eisai: Consultancy; Genzyme: Consultancy; 3SBios: Consultancy; Shire: Consultancy; Amgen: Consultancy, Paid expert testimony; Pfizer: Consultancy; Shionogi: Consultancy; ONO: Consultancy; GlaxoSmithKline: Consultancy; Bristol-Myers Squibb: Research Funding; Protalex: Research Funding; CRICO: Other: Paid expert testimony; Rigel: Consultancy, Research Funding. Francovitch:Protalex, Inc.: Employment. Michel:Amgen: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal