Abstract
Introduction
Hematologic neoplasms are associated with an increased incidence of autoimmune events, particularly evident in non‐Hodgkin lymphomas (NHL) and especially chronic lymphocytic leukemia (CLL); until now only few cases have been described in patients affected by multiple myeloma (MM) and other plasma cell disorders. The introduction of new drugs, in particular immunomodulatory drugs (IMiDs), could increase the risk of these complications.
Herein we describe four cases of immune thrombocytopenia (ITP) occurred during Lenalidomide (LEN) therapy in 3 patients affected by MM and 1 with light‐chain amyloidosis.
Case reports
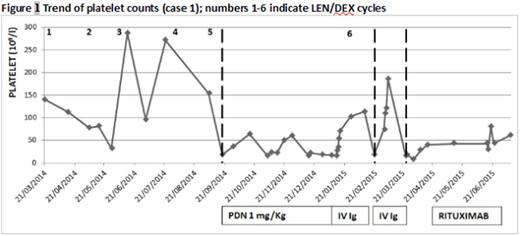
Case 1. A 66‐year old woman with relapsed IgGk MM started 2th line treatment with LEN/DEX 25 mg/d-20 mg/w. During the first 5 cycles she presented moderate hematologic toxicity which resolved after dose reduction (LEN 15 mg/d). A severe and isolated thrombocytopenia appeared after 5th cycle with patient in good partial remission (PR) for MM. A bone marrow evaluation showed absence of plasma cells and abundant megakaryocytes leading to a diagnosis of ITP. LEN was interrupted and steroid therapy (prednisone 1 mg/Kg) and IV Ig infusion administered, obtaining a transient good response (from 17 to 114 x 109/l). LEN was then restarted at lower dosage but a month after, while myeloma still was in good response, ITP relapsed complicated by intracranial hemorrhage and was successfully treated with IV Ig; Len was definitely interrupted. At the third relapse Rituximab (750 mg/w x 4 weeks) was administered with only minimal increase of platelet count (Fig 1). Afterwards, the patient died due to rapid MM progression.
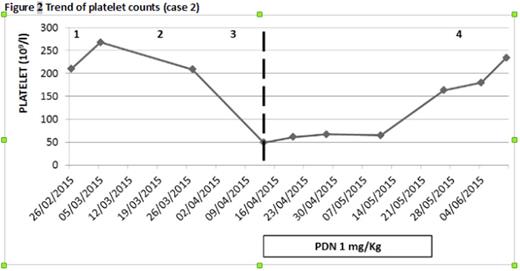
Case 2. A 76‐year old woman with relapsed IgGλ MM started 2th line treatment with LEN 15 mg. After the 3rd cycle, having obtained a very good partial remission (VGPR), she presented with grade 3 thrombocytopenia which did not ameliorate with LEN suspension and resolved only after introduction of steroid, thus supporting ITP diagnosis. She underwent a 4th cycle of LEN/DEX maintaining a normal platelet count but then LEN was interrupted due to rapid MM progression. (Fig 2).
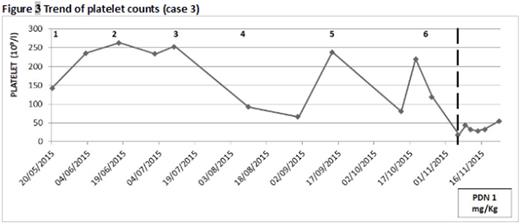
Case 3. A 78‐year old woman with renal amyloidosis in IgAλ gammopathy started a 2nd line treatment with LEN/DEX 15 mg/d-20 mg/w with a significant reduction in proteinuria. During the 6th cycle she presented with diffuse purpura and a platelet count of 18x109/L. She received platelet transfusion and bone marrow evaluation showed abundant megakaryocytes supporting the diagnosis of ITP. Furthermore anti‐platelet antibodies search resulted positive. Steroid therapy was started with partial response on platelet count but the patient remained steroid‐therapy dependent.
Case 4. A 66‐year‐old woman affected by MM started a 2th‐line treatment with LEN‐DEX 25 mg/d-20 mg/w. After the third cycle, being the patient in PR, a isolated grade 3 thrombocytopenia developed, persisting despite discontinuation of LEN. Viral infections were excluded and antiplatelet antibodies search resulted positive. Therefore she started steroid therapy (prednisone 1 mg/Kg) with an progressive increase of platelet count from 44 to 80x109/L after a month of therapy, that is ongoing. Lenalidomide has not yet been restarted.
Discussion
We report four cases of ITP developing during LEN therapy with the characteristics of ITP. None of these patients had a history of previous autoimmune events, the decline of platelet count was rapid and other causes of thrombocytopenia were excluded or unlikely in all the cases. Furthermore diagnosis was supported by consistent bone marrow evaluation in two cases. Considering the temporal association in these four cases, the ITP mechanism may be related to the immunomodulation and T‐cell activation caused by LEN.
Even if further other studies are needed, it seems reasonable to consider a possible association between LEN and autoimmune phenomena, in particular ITP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal