Abstract
Introduction: Nearly 40% of adolescent women experience heavy menstrual bleeding (HMB), and identifiable bleeding disorders are diagnosed in only 20-60% of these patients. We suspect that qualitative platelet disorders contribute to HMB, but are under-diagnosed. A pilot study was conducted to evaluate platelet function in adolescent women with HMB employing four novel, small-volume, whole blood platelet function assays. In addition, primary and secondary hemostasis, bleeding phenotype, and quality of life were assessed.
Methods: Patients referred to the Young Women's Hematology Clinic at Oregon Health & Science University for evaluation of HMB were offered participation in the study. Participants underwent standard review of their medical and family history and physical exam. Standard lab evaluation included CBC, PT, PTT, fibrinogen, thrombin time, Von Willebrand Panel, PFA-100, and iron studies with platelet aggregation or phenotyping performed if clinically indicated. Using less than 0.5 mL of whole blood, platelet function was assessed with four novel platelet function assays: assessment of platelet activation, secretion, and aggregation was assessed by flow cytometry analysis, while platelet adhesion and aggregation was assessed under shear in a capillary tube. Quality of life (QOL) was assessed using the PedsQL tool. Bleeding phenotype was assessed with the ISTH Bleeding Assessment Tool (ISTH BAT). Menorrhagia was assessed with the Pictorial Bleeding Assessment Chart (PBAC), the Philipp Tool and the clinical history.
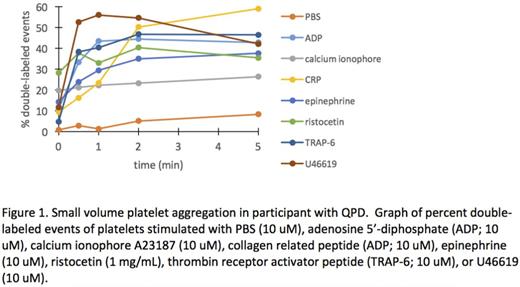
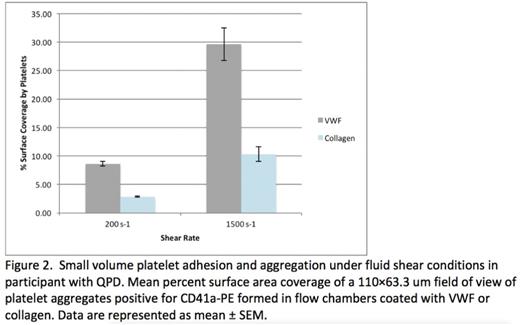
Results: Nine participants have enrolled on study to date, with 2 completing the 3-month visit. The median age of the cohort was 16 years (14-18 years). Eight out of nine categorized their period as heavy, 6 also had epistaxis, and 7 reported excessive bruising. The median ISTH BAT score was 4 (3-7). Of the 7 patients who had a Philipp Score obtained, 5 were positive. Median PBAC score was 161 (64-196). Median ferritin was 13 ng/mL (4-65 ng/mL). Median QOL psychosocial score was 70 (68.36-88.25), comparable to that of pediatric patients with cancer. Of the 9 participants, 6 had platelet aggregation and phenotyping. Four participants did not receive a bleeding disorder diagnosis, 1 was diagnosed with Type 1 VWD, 1 was diagnosed with bleeding disorder, NOS, and 1 was diagnosed with Ehlers Danlos Syndrome. Two participants were diagnosed with a qualitative platelet disorder (QPD): one based on platelet aggregation and one based on thromboelastography. The four novel platelet function assays confirmed platelet function abnormalities in the participants diagnosed with QPD's (Figure 1&2). Impaired platelet response to agonist stimulation was also observed in participants with non-platelet disorder bleeding disorder diagnoses and in participants without a bleeding disorder diagnosis.
Conclusions: In this pilot study, the etiology of HMB in adolescent women was evaluated with four novel platelet assays in addition to standard assays of hemostasis. A bleeding disorder diagnosis was not made with standard evaluations in 4 out of 9 participants. The novel assays detected platelet abnormalities not observed using currently available clinical labs, and confirmed the presence of abnormal platelet function in participants with abnormal platelet function testing. These assays require significantly less blood volume than currently available assays and expand investigation of platelet function to platelet adhesion and platelet interactions in whole and flowing blood. Further work is needed to determine the sensitivity and specificity of the novel assays in detecting platelet dysfunction. Continued investigation into the impact of HMB on the adolescent female population is needed.
Haley:CSL Behring: Honoraria; Baxalta: Membership on an entity's Board of Directors or advisory committees. Recht:Biogen: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Biogen: Research Funding; Genentech: Research Funding; Novo Nordisk: Research Funding; Baxalta: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal