Abstract
Background: Romiplostim is a subcutaneously administered thrombopoietin-receptor agonist approved for use in the EU for adult patients with chronic immune thrombocytopenia (ITP) refractory to other treatments. Initially approved for administration by healthcare providers (HCP), romiplostim was further approved by the European Medicines Agency in December 2012 for self-administration by select patients/caregivers. Romiplostim-treated chronic ITP patients with stable platelet count ≥ 50 x 109/L for ≥ 4 weeks without dose adjustment eligible for self-administration should be trained in self-administration procedures, and be supervised again after the first 4 weeks of self-administration while reconstituting and injecting romiplostim. To mitigate medication error risk due to self-administration, an additional risk minimisation activity in the form of a Home Administration Training (HAT) pack was designed to support HCPs in selecting patients and training of patients/caregivers. For subcutaneous self-administration of romiplostim, each patient (or caregiver) is supplied with self-administration kit (250/500 mcg romiplostim vial, plunger rod, prefilled sterile water syringe, disposable syringe with Luer-lok, safety needle, vial adapter) and a HAT pack, including placemat to lay out the administration components, checklist, instructions for use, self-administration diary, and instructional video.
Aims: To estimate the proportion of adult patients and caregivers who administered romiplostim correctly after being trained with HAT pack.
Methods: This multicentre, non-interventional, cross-sectional study was conducted at 12 study centres across 8 countries (Austria, Belgium, France, Germany, Greece, The Netherlands, Spain, and The United Kingdom) from 07 July 2014 to 20 November 2015. Adult (≥ 18 years of age) chronic ITP patients prescribed romiplostim self-administration or caregivers new to administering romiplostim, who provided informed consent at beginning of HAT pack training, received HAT pack training, and were available at first standard-of-care 4-week visit after training, were consecutively enrolled in the study. HCPs directly observed the patient/caregiver in the act of administering romiplostim at the first standard-of-care 4-week visit after HAT pack training, and completed a standardized data collection form. Variables collected included patient demographics, prescribed and administered injection volume per syringe, appropriate alcohol wipe use at injection site, clinically appropriate handling of syringe to avoid contamination, and clinically appropriate subcutaneous injection technique. The primary endpoint, correct administration of romiplostim, was defined as romiplostim dose accuracy within 10% margin of error between prescribed and administered romiplostim dose, and correct romiplostim reconstitution, and successful romiplostim injection, and no HCP intervention during administration to correct patient/caregiver error. All analyses were descriptive and no formal hypothesis was tested.
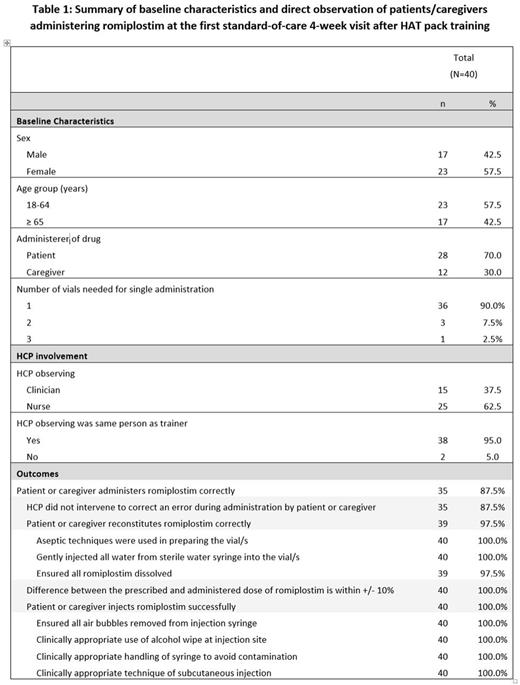
Results: A total of 40 patients/caregivers were enrolled in the study (Table 1). At the first standard-of-care visit, 4 weeks (range: 2 - 8 weeks) after HAT pack training, 35 patients/caregivers (87.5%) administered romiplostim correctly. Romiplostim dose accuracy was within 10% margin of error for all patients. HCP intervention was required in 5 instances (3 patients, 2 caregivers): 1 patient did not ensure all romiplostim was dissolved, 1 patient and 1 caregiver needed verbal encouragement, 1 patient needed nursing intervention to read the correct dose from the vial due to poor eyesight, and 1 caregiver needed guidance with syringe and vial connection. Further follow-up data was available for 2 of these 5 patients/caregivers; they both administered romiplostim correctly at a subsequent visit.
Conclusion: Given that this study was conducted on a convenience instead of random sample of patients, generalizability of the results may be limited. Direct observation can be susceptible to observation bias and to the Hawthorne effect with the patients/caregivers acting differently when observed. Nonetheless, the success of most patients and caregivers in correctly administering romiplostim after HAT pack training suggests that self-administration of romiplostim is a feasible option for suitable romiplostim-treated ITP patients.
Schipperus:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Partial sponsorship(50% of costs)ASH 2015. Taylor:Amgen: Consultancy. Wetten:Amgen: Employment, Equity Ownership. Bennett:Amgen: Employment. Kreuzbauer:Amgen: Employment, Equity Ownership. Boshier:Amgen: Employment, Equity Ownership. Seesaghur:Amgen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal