Abstract
Background: Among the three phenotypes of Gaucher disease (GD), type 1 is the most prevalent in the Western hemisphere, but types 2 and 3 are increasingly seen, and occur with similar prevalence to type 1 in parts of Asia. There is a spectrum of signs and symptoms among these phenotypes, which range from fatal perinatal to asymptomatic adult disease, and this heterogeneity contributes to relatively high levels of misdiagnosis and delays in diagnosis of GD. As part of the global Gaucher earlier diagnosis consensus (GED-C) initiative, we report here the signs and patient co-variables that are regarded by expert physicians as most indicative of type 3 GD in its early stages. The overarching goal of the GED-C initiative was to generate a web-based point-scoring system for use across clinical specialties to facilitate identification of patients who may benefit from diagnostic testing for GD.
Methods: In an anonymous, iterative Delphi process, a panel of expert physicians was asked to provide free-text answers to a series of open questions, including: "Which unexplained signs and co-variables may be important to consider in early type 3 GD?" An independent facilitator categorized responses from round 1 into themes, which were checked and consolidated by the two non-voting co-chairs of the initiative to generate a set of summary factors. In round 2, panel members independently rated the importance of each factor using a 5-point Likert scale (1 = not important, 5 = extremely important). Factors that were assigned an importance score of at least 3 by more than 75% of respondents were provisionally classified as major; other factors were classified as minor. In round 3, panel members rated their level of agreement with the provisional classification of factors as major using a 5-point pivoted Likert scale (1 = strongly disagree; 3 = neither agree nor disagree; 5 = strongly agree). Consensus was defined as more than 67% of respondents agreeing or strongly agreeing (a score of ≥ 4) with the classification; if consensus was not reached, factors were classified as minor.
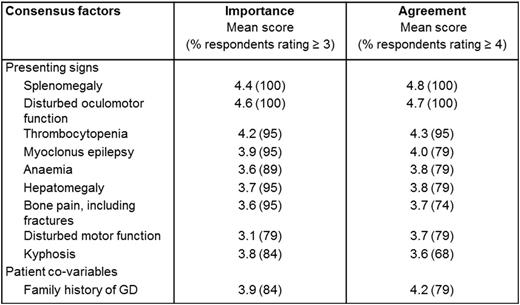
Results: In total, 19 physicians with expertise in type 3 GD were recruited to the GED-C panel from 14 countries. Round 1 (100% response, n = 19) yielded 70 phrases, which were grouped into 34 themes, then consolidated as 23 factors. In round 2 (100% response, n = 19), 16 factors were provisionally classified as major. In round 3 (100% response, n = 19), consensus was reached on 10 major factors in early type 3 GD, including 9 presenting signs and 1 patient co-variable. The mean importance scores (round 2) and agreement scores (round 3) awarded to these 10 major factors are given in Table 1. Minor factors included bleeding or bruising, cardiovascular calcification, cognitive deficit, growth retardation, hyperferritinaemia, aged 18 years or younger, Jewish ancestry and a family history of Parkinson disease.
Discussion: Definitive diagnostic tests for GD have been available for several years, but patient referral for testing is impeded by several issues, including a lack of knowledge among clinicians of the signs and co-variables that should arouse suspicion of GD. In type 3 GD, the problem is exacerbated by the relative rarity of the phenotype. Presenting signs and patient co-variables identified by this multidisciplinary consensus initiative will help clinicians identify those patients who may benefit from diagnostic testing for GD. Several algorithms have been devised to facilitate GD diagnosis, but non-specialists may perceive these as complex. The GED-C initiative will use the factors identified here to create a point-scoring system that clinicians of any specialty can use to obtain clear direction regarding the need to test a patient for GD.
Acknowledgment: Submitted on behalf of the GED-C panel members and the European Hematology Association Scientific Working Group 'Quality of Life and Symptoms'. Administration of the GED-C initiative was funded by an unrestricted educational grant from Shire.
Mean scores of importance and agreement for 10 major factors in early diagnosis of type 3 Gaucher disease.
Mean scores of importance and agreement for 10 major factors in early diagnosis of type 3 Gaucher disease.
Kuter:Eisai: Consultancy; Genzyme: Consultancy; MedImmune: Consultancy; Rigel: Consultancy, Research Funding; CRICO: Other: Paid expert testimony; Pfizer: Consultancy; Protalex: Research Funding; GlaxoSmithKline: Consultancy; Amgen: Consultancy, Paid expert testimony; Bristol-Myers Squibb: Research Funding; ONO: Consultancy; Shionogi: Consultancy; Shire: Consultancy; Syntimmune: Consultancy; 3SBios: Consultancy. Salek:Novartis: Research Funding; Bristol-Myers Squibb: Research Funding; Shire: Consultancy; Servier: Consultancy; Sanofi: Research Funding; Agios: Consultancy. Mehta:Protalix/Pfizer: Honoraria, Other: travel grant, Research Funding; Genzyme: Honoraria, Other: travel grant, Research Funding; Actelion: Honoraria, Other: travel grant, Research Funding; Shire: Honoraria, Other: travel grant, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal