Background:
Hemophagocytic Lymphohistiocytosis (HLH) is an often-fatal syndrome of uncontrolled immune activation caused by dysregulation of macrophages and lymphocytes resulting in increased levels of inflammatory cytokines and significant tissue damage. Familial HLH (FHL) is characterized by germline mutations in genes affecting the cytolytic function of Natural Killer (NK) cells and cytotoxic T cells. Secondary HLH is triggered by an infectious, rheumatologic, malignant or autoimmune condition. While FHL is well characterized in the pediatric population, there are currently no available prospective data guiding the diagnosis and treatment of secondary HLH in the adult patient. Here we present 7 cases of adult secondary HLH that were identified and treated at Weill Cornell Medical College in a period of two years. We highlight the presentation and etiology of secondary HLH and the importance of prompt recognition and early treatment. We also explore the relationship of immunosuppressive and antiretroviral therapy with adult secondary HLH.
Methods:
Between July 2014 and July 2016, 7 adult patients >18 years old were identified at Weill Cornell Medical College, NY that met criteria and were treated for adult secondary HLH. All 7 patients had 5 out of 8 of the following findings established in the HLH-2004 guidelines: fever ≥ 38.5 C°, splenomegaly, cytopenias affecting 2 of 3 lineages in the peripheral blood (hemoglobin <9 g/dL, platelets <100x103/mL, neutrophils <1x103/mL), hypertriglyceridemia (fasting >265 mg/dL) and/or hypofibrinogenemia (<150 mg/dL), hemophagocytosis in the bone marrow, spleen, lymph nodes or liver, low or absent NK cell activity, ferritin >500 ng/mL and elevated sIL2R. Molecular testing was sent to identify pathologic mutations associated with FHL. Patients were treated with chemotherapy and immunotherapy based on the pediatric HLH-94 protocol, as well as treatment targeted at an underlying malignancy or infection. Patient demographics and clinical characteristics, underlying etiology of secondary HLH and treatment modalities were compared.
Results:
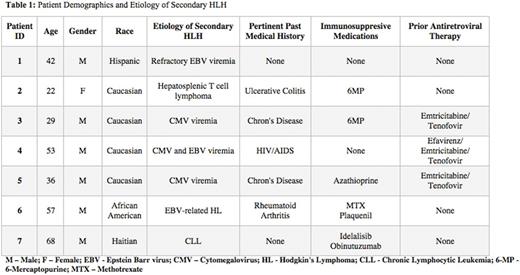
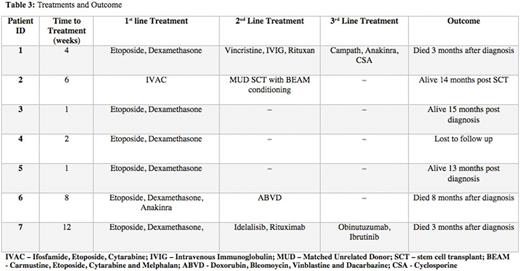
All 7 patients included in this study met criteria for secondary HLH as defined above. The median age at diagnosis was 42 (range 22-68 years) with a male predominance of 6:1. The majority of patients identified as Caucasian (4/7, 57%) and the remaining 3 patients were Hispanic, African American and Haitian. Five out of 7 (71%) patients had been on immunosuppressive therapy prior to diagnosis, 3 patients had Inflammatory Bowel Disease (IBD) and 3 patients were on Emtricitabine and Tenofovir at the time of diagnosis. The median ferritin at diagnosis was 4,589 ng/mL (range 2,127-10,500 ng/mL) with a peak median ferritin of 10,500 ng/mL (range 3,093-306,320 ng/mL). The sIL2R at diagnosis ranged from 1,000-112,200 pg/mL (reference <1,033 pg/mL). Patient clinical manifestations are summarized in Table 2. All patients presented with fevers, elevated ferritin, cytopenias and splenomegaly and 6/7 (86%) patients had evidence of hemophagocytosis in the bone marrow or liver. Four patients (57%) had secondary HLH due to an infection and 3 (43%) had an underlying malignancy. All patients received Etoposide and Dexamethasone and 4 patients received 2ndline treatment either for an underlying malignancy or for refractory HLH. The median time from presentation to initiation of treatment was 4 weeks (range 1-12 weeks). At the time of this report, 3 patients had died (43%) of which 2 had an underlying malignancy, 3 were alive and 1 patient was lost to follow up.
Discussion:
Diagnosis of adult secondary HLH remains a challenge due to overlap in symptoms and lack of specificity of laboratory findings. Treatment is often postponed due to delayed recognition of this disease. A high degree of medical suspicion in the appropriate clinical context should prompt early treatment with an Etoposide-containing regimen. Interestingly, we found 4 patients with autoimmune conditions on immunosuppressive therapy, 2 of which were also on antiretroviral treatment. Further investigation is needed to elucidate the association of these findings in adult secondary HLH. Molecular mutational testing is of interest in identifying possible underlying genetic predisposition for adult secondary HLH and further studies are warranted in this area.
Ritchie:Celgene: Consultancy, Other: Travel, Accomodations, Expenses, Speakers Bureau; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Ariad: Speakers Bureau; Pfizer: Consultancy, Research Funding; Astellas Pharma: Research Funding; Bristol-Meyers Squibb: Research Funding; NS Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal