Abstract
Background: Adequate iron chelation therapy (ICT) and patient adherence are essential in the management of patients (pts) with chronic iron overload (IOL), which is an important cause of mortality and morbidity. Data from various clinical trials show that deferasirox (DFX), a once-daily oral iron chelator, at a recommended dose of 20 mg/kg/d stabilizes serum ferritin (SF) and liver iron concentration in transfusion-dependent pts (Chaudhary et al. J Blood Med. 2013;4:101-10). The non-interventional, SENTINEL study conducted in pts with transfusional hemosiderosis demonstrated the long-term safety and efficacy of DFX. The current analysis was performed to further evaluate the safety and efficacy of DFX in pts who completed the 3-year (yr) study, including pts who completed the study and received an overall average actual dose of DFX ≥20 mg/kg/d.
Methods: The SENTINEL study design has been reported previously (El-Beshlawy et al. Haematologica 2016;101(s1):abst E1475). Pts aged ≥2 yrs with transfusional hemosiderosis treated with DFX in accordance with the local prescription information were enrolled. For this analysis, pts who completed the 3-yr study and/or completed the 3-yr study receiving an overall average actual dose of DFX ≥20 mg/kg/d were considered. Safety was assessed by regular monitoring and recording of adverse events (AEs) and efficacy was measured by SF levels, which were analyzed biannually, by providing summary statistics for the average SF values of each pt within that specific period.
Results: Of the 120 pts enrolled, 51 (42.5%; <18 yrs, n=45; ≥18 yrs, n=6) with β thalassemia (n=30); sickle cell disease (SCD, n=18) and other anemias (n=3) completed the 3-yr study. 41/51 pts (80.4%; <18 yrs, n=37; ≥18 yrs, n=4) with β thalassemia (n=26), SCD (n=12), and other anemias (n=3) received an overall average actual dose of DFX ≥20 mg/kg/d. The most frequent reasons for study discontinuation (>10%) include pts no longer required study drug (19.2%), AEs (12.5%), and consent withdrawn (10.8%).
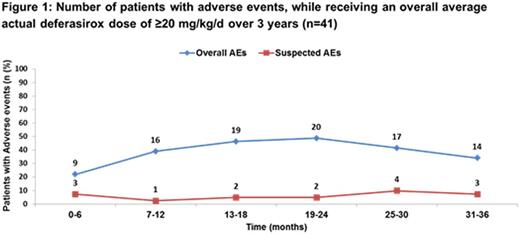
Of the pts who completed the study, 14 (27.4%) had AEs suspected to be related to DFX, which included 10 pts receiving an overall average actual dose of DFX ≥20 mg/kg/d. The most frequent AEs (>5%) suspected to be related to DFX in pts who completed the 3-yr study were vomiting (7.8%), abdominal pain (5.9%) and lack of efficacy (5.9%). The overall incidence of AEs with suspected relationship to DFX, in pts who completed the study and received an overall average actual dose of DFX ≥20 mg/kg/d was consistent throughout the study without any unexpected safety findings (Figure 1).
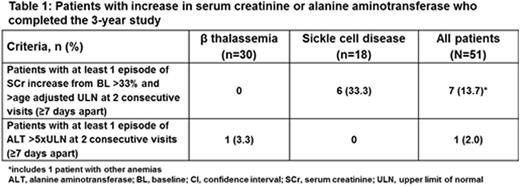
Increase in serum creatinine, >33% above baseline (BL) and the age-adjusted upper limit of normal (ULN) on at least 2 consecutive measurements (≥7 days apart), was observed in 7 (14.3%) pts who completed the study (Table 1), of whom 6 (SCD, n=5; other anemias, n=1) received an overall average actual dose of DFX ≥20 mg/kg/d.
Increase in alanine aminotransferase (ALT), >5×ULN on at least 2 consecutive measurements (≥7 days apart), was observed in 1 pt with β thalassemia (BL ALT missing) during the last yr of study (Table 1). No notable increase in ALT was observed in pts, who received an overall average actual dose of DFX ≥20 mg/kg/d.
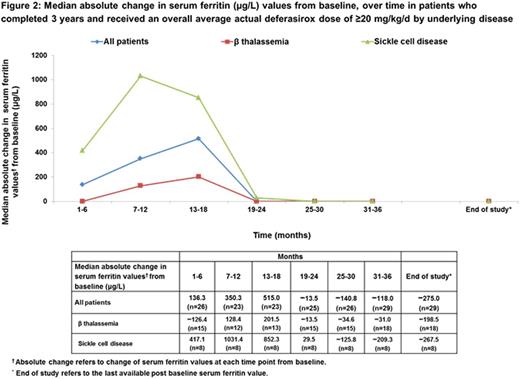
In pts who completed the study, the median absolute change in SF (µg/L) values from BL to last available post BL (end of study [EOS]; n=37) was −275.0. In pts with β thalassemia and SCD, the median absolute change in SF values from BL to EOS were −284.0 (n=21) and −260.0 (n=13) respectively.
In pts who completed the study and received an overall average actual dose of DFX ≥20 mg/kg/d, the median absolute change in SF values from BL to EOS (n=29) was −275.0 (Figure 2).
Conclusions: Most of the pts who completed this 3-yr study had a diagnosis of β thalassemia and SCD. Overall, 42.5% of pts received DFX treatment for 3 yrs, and the majority of them were treated with an overall average actual dose of DFX ≥20 mg/kg/d. The observed safety profile of DFX was consistent with the previously published data. In pts who completed the study and received a dose of DFX ≥20 mg/kg/d, few had AEs suspected to be related to DFX. In pts who completed the study, the median change in SF values decreased from BL to EOS. The results demonstrate that an average actual dose of DFX ≥20 mg/kg/d might be required in the majority of pts, with appropriate dose adjustments based on iron intake and IOL to ensure an effective ICT, particularly in pediatric pts with transfusional hemosiderosis.
Bruederle:Novartis Pharmaceuticals Corporation: Employment. Azmon:Novartis Pharmaceuticals Corporation: Employment. El-Beshlawy:Novartis Pharmaceuticals Corporation: Research Funding; Apo Pharma Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal