Abstract
Background: Autoimmune hemolytic anemia (AIHA) is a complication of pediatric hematopoietic stem cell transplantation (HSCT) with an incidence of 2% following first transplants and almost 10% after second transplants (Ahmed et al. Ped Transplant 2015). The specific cause is unknown but is thought to be related to immune dysregulation. We present the case of a patient who developed post-HSCT AIHA which was refractory to multiple conventional therapies, but finally responded to therapy with the anti-CD38 monoclonal antibody daratumumab.
Case: The patient is a 19-year-old female with high risk pre-B acute lymphoblastic leukemia (ALL) who was treated per AALL1131 and was minimal residual disease (MRD) positive by deep sequencing after delayed intensification. She underwent an 11/12 DP-mismatched unrelated donor peripheral blood stem cell transplant after myeloablative conditioning with alemtuzumab, busulfan, fludarabine and clofarabine. Because of persistently positive MRD and declining donor CD3 chimerism, she received azacitidine and a donor lymphocyte infusion 2 months later. One month later, MRD was negative but she then developed severe AIHA, presenting with a hemoglobin of 4.7 g/dL, strong Coombs positivity demonstrating reactive anti-E, elevated lactate dehydrogenase (LDH) and hemoglobinuria. She required 11 units of packed red blood cells (pRBCs) in the first 10 days of her illness. All subsequent pRBC transfusions were E-antigen negative.
She was treated with high-dose methylprednisolone, rituximab, and bortezomib with temporary response. Because of persistent MRD and recurrent hemolysis, she was then treated with high-dose cyclophosphamide, rabbit anti-thymocyte globulin and a stem cell boost 2 months after her presentation with AIHA, with only transient effect. Over the following 14 months, she had recurrent episodes of severe hemolysis prompting therapy with multiple additional agents (including high-dose methylprednisolone, an additional course of rituximab, alemtuzumab, bortezomib, mycophenolate mofetil, sirolimus, and ibrutinib). Splenectomy was deferred due to a history of recurrent encapsulated organism bacteremia / sepsis. During this time, her direct antiglobulin test remained strongly positive; she received pRBC transfusions at least twice weekly despite all therapies, requiring a total of 200 units of pRBC's following the onset of AIHA.
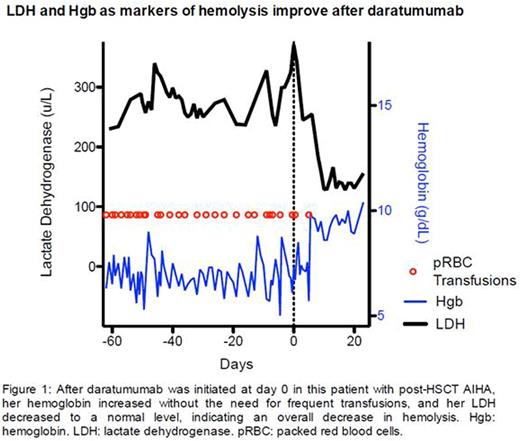
We hypothesized that ongoing Coombs positivity despite B cell depletion indicated that a residual population of host plasma cells was producing anti-donor RBC antibodies. Since CD38 is highly expressed on plasma cells, we therefore administered the anti-CD38 IgG1κ human monoclonal antibody daratumumab. She received standard multiple myeloma dosing (16mg/kg IV once weekly with pre-medications including methylprednisolone and 2 days of prednisone following the infusion to prevent hypersensitivity reaction, Lonial et al. Lancet 2016). She required one additional pRBC transfusion 5 days following the first infusion. Her hemoglobin subsequently increased without transfusion and her LDH normalized for the first time in over a year. At 4 weeks following her first dose of daratumumab, the strength of her Coombs test has decreased but has remained moderately positive; haptoglobin has remained undetectable. Given her medical complexity, it is difficult to assess side effects, but no adverse events have been directly attributed to treatment with daratumumab.
Conclusion: In our patient with post-HSCT AIHA who was refractory to multiple therapies, daratumumab dramatically decreased her pRBC transfusion requirement; laboratory parameters indicate this was a result of decreased active hemolysis. Further study is warranted to assess whether CD38 targeting is a safe and effective treatment for patients with refractory post-HSCT AIHA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal