Abstract
Introduction
High dose chemotherapy with or without radiation followed by hematopoietic stem cell transplantation (HSCT) is standard therapy for pediatric patients with very high risk acute leukemia. Despite maximal therapy, a significant number of these patients will relapse after HSCT (Bitan Blood 2014; Tracey BBMT 2013). The hypomethylating agent azacitidine has immunomodulatory activity at a low dose and may augment anti-leukemic graft alloreactivity when used after HSCT (Goodyear Blood 2012; Craddock BBMT 2016). We report on the feasibility and safety of azacitidine administration following HSCT for pediatric acute leukemia.
Methods
Enrollment of pediatric patients with acute leukemia in this phase I/ II prospective study (NCT02458235) started in May of 2015 and is ongoing. Intravenous azacitidine (160 mg/m2 divided over 4 days) was administered as early as Day +30 post-HSCT for up to 7 consecutive cycles. Patients underwent myeloablative conditioning regimens according to standard protocols using a backbone of either total body irradiation or busulfan, combined with one or more of the following: thiotepa, melphalan, fludarabine, and clofarabine. Any stem cell source was allowed.
Azacitidine was combined with immunomodulatory interventions such as fast withdrawal of immunosuppression and donor lymphocyte infusion if indicated, based on mixed chimerism or persistent minimal residual disease. Study subjects were monitored for azacitidine toxicity via weekly blood tests (complete blood count and biochemistry panel). Lymphocyte subsets and function were tested prior to each azacitidine cycle. Azacitidine was not administered in cases of hepatic injury as measured by elevated transaminases >5 times upper limit of normal; cytopenias requiring regular blood product transfusions or growth factor support; vital sign instability; or at the discretion of the treating physician. If tolerated, azacitidine was continued in 6-weekly cycles for a maximum of 7 cycles.
Results
Of 11 patients registered on the study with intent to receive azacitidine, 6 received the first dose of azacitidine prior to Day +60 (ranging from day +38 to day +60). An additional 4 patients received the first dose of azacitidine when clinically stable (ranging from Day +67 to Day +95), while 1 patient did not receive the drug due to early death from infection following HCT. Following azacitidine administration, thrombocytopenia requiring transfusion (10 x 109/L) was observed in 1 patient; anemia requiring transfusion (Hb 6.4 g/dL) in 1 patient; and transient elevated LFT's > 5 times the upper normal limit in 4 patients which resolved spontaneously. An additional 2 patients developed sustained elevation in LFT's related to GVHD and no further azacitidine was administered.
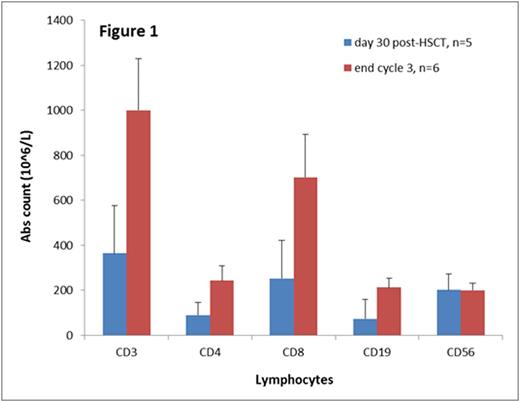
No patients required delays in subsequent cycles by > 4 weeks due to side effects of azacitidine. One patient developed VOD at Day +50 post-HSCT following azacitidine administration and did not receive further cycles of azacitidine. One patient developed Grade 4 acute GVHD and went on to develop severe chronic GVHD. An additional 3 patients developed Grade 2-3 acute GVHD which resolved in two of these. At a median follow up of 8 months, 2 patients had experienced leukemia relapse. All of the 6 patients followed for >6 months achieved T cell reconstitution with CD4 and CD8 counts >200 (Figure 1). Five of these 6 patients achieved CD3 phytohemagglutinin response > 50% of control; one patient did not due to treatment for chronic GVHD.
Conclusion
Low-dose azacitidine administration is feasible in the post-HSCT setting for pediatric patients with acute leukemia. No severe toxicity directly attributable to azacitidine was observed; the role of azacitidine in the development of late VOD in one patient is unclear. Although transient liver abnormalities and cytopenias were common, low-dose azacitidine appears to be safe in the immediate post-transplant period. Further studies of immunomodulatory effects of azacitidine are required in order to optimize post-HSCT graft alloreactivity and minimize the risk of post-HSCT relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal