Abstract
Background: ECD and LCH are rare disorders for which no approved therapies are available. BRAFV600E mutations have been observed in 50% of patients with LCH and in 50-60% of patients with ECD. Here we present data from a planned Week 16 analysis of patients with ECD/LCH who were enrolled in the VE-BASKET study (ClinicalTrials.gov identifier NCT01524978).
Methods: This open-label, Simon 2-stage adaptive-design, phase 2 study included patients with BRAFV600E-mutant ECD and LCH. Patients received vemurafenib (960 mg bid) until disease progression or unacceptable toxicity. The primary endpoint was investigator-assessed response rate at Week 8; secondary endpoints included best overall response rate, clinical benefit rate, progression-free survival (PFS), and overall survival (OS). For a subset of patients (n=15), metabolic response by 18F-FDG-PET was assessed; five target lesions were selected and their maximal standardized uptake value (SUVmax), normalized for body weight (BW), was compared with background (liver) SUV. Data cut-off was 2 October 2015.
Results: 26 patients (22 with ECD and 4 with LCH; median age 61y) were enrolled. Seven patients (27%) had one prior therapy, six (23%) had two prior therapies, four (15%) had ≥3 prior therapies, and nine (35%) had no prior systemic therapy. Six patients were in follow-up at data cut-off; 16 were on treatment.
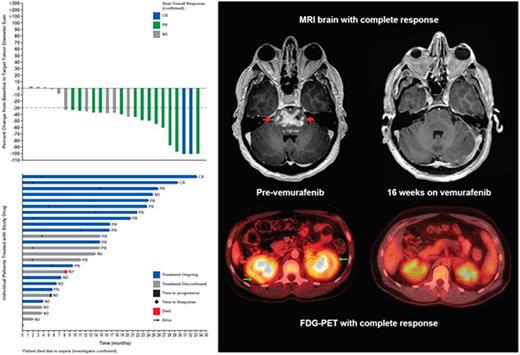
Best overall response (according to RECIST v1.1) in 25 patients with measurable disease at baseline was 60% (95% CI 38.7-78.9%) with complete response (CR) in two patients (8%) and partial response (PR) in 13 patients (52%) (Figure). Responses were seen in patients with ECD (1 CR, 10 PRs) and LCH (1 CR, 3 PRs). After a median treatment exposure of 14.2 months (range 4.2-22.5 months), median OS and PFS have not been reached.
All of the 15 patients assessed by 18F-FDG-PET showed a response: 12 patients had a complete metabolic response (normalization of all lesions' SUVmax-BW to background SUV) and three had a partial metabolic response (>50% decrease in sum of baseline SUVmax of all target lesions) (Figure).
Overall, safety data were consistent with prior studies of vemurafenib. Arthralgia (n=17; 65%), fatigue (n=15; 58%), rash macropapular (n=14; 54%), alopecia (n=14; 54%), skin papilloma (n=14; 54%), prolonged QT (n=12; 46%), and palmar-plantar erythrodysesthesia syndrome (n=12; 46%) were the most common all-grade adverse events (AEs). Seventeen patients had serious AEs, including 10 with squamous cell carcinoma of the skin. Seven patients discontinued vemurafenib due to AEs.
Conclusion: These data, which represent the only prospective clinical trial data of BRAF inhibition in histiocytosis to date, reveal that vemurafenib has potent single-agent activity in patients with ECD/LCH. Moreover, vemurafenib treatment had remarkable durability of response in histiocytosis, such that no evidence of resistance has been encountered following a median of 14.2 months of treatment. These results are distinct from vemurafenib use in other solid or hematologic malignancies.
Subbiah:Abbvie: Research Funding; Nanocarrier: Research Funding; GlaxoSmithKline: Research Funding; Roche/Genentech: Research Funding; Bayer: Research Funding; Novartis: Research Funding. Blay:F. Hoffmann-La Roche: Consultancy, Research Funding; MDS: Research Funding; Lilly: Research Funding; Bayer: Consultancy, Research Funding; Pharmamar: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Puzanov:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunocore: Consultancy. Wolf:F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Clovis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer-Ingelheim: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ulaner:GE Healthcare: Research Funding; Genentech: Research Funding; Blue Earth Diagnostics: Research Funding; Susan Komen Foundation: Research Funding; Department of Defense: Research Funding; National Institutes of Health: Research Funding; Zevacor: Honoraria. Lacouture:Quintiles: Consultancy; Boehringer Ingelheim: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy; Foamix: Consultancy; Infinity: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Berg: Research Funding; Bristol Myers Squibb: Research Funding. Robson:F. Hoffmann-La Roche Ltd: Employment. Makrutzki:F. Hoffmann-La Roche Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal