Abstract
Introduction
MMF is an immunosuppressive drug frequently used for graft-versus-host disease (GVHD) prophylaxis and management. MMF-induced colitis is a known complication, however, details regarding its clinical manifestations, treatment options, and outcomes are less clear. Differentiating MMF-induced colitis from gut GVHD on the basis of histology may be difficult, hence deeper clinical understanding of MMF-induced colitis can be valuable. The aim of this systematic review was to analyze the reported cases of MMF-induced colitis to provide summative data.
Methods
Inclusion criteria included prospective or retrospective clinical studies and case series, reported in English language, providing data on clinical manifestations, treatment options, and outcomes of colitis induced by MMF or its derivatives (such as mycophenolic acid, and enteric coated mycophenolate sodium). Cases were included irrespective of the indication of MMF. Using various search terms, all cases indexed in PubMed, EMBASE, Cochrane and Scopus from inception to July 2016 were reviewed. The bibliography of each relevant article was searched for additional reports. Non-human studies and colitis attributed to other etiologies such as infection, GVHD or inflammatory bowel disease were among the 138 articles that were excluded.
Results
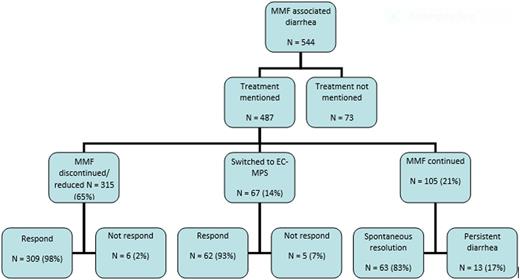
Forty-four articles with a total of 544 patients and 560 episodes of suspected or confirmed MMF-induced colitis were included. The median age was 41 years (range 5-70), and 30% were females. The latency between the use of MMF and onset of colitis was 990 days (range 3- 5760). Watery diarrhea as frequent as every 20 minutes in one case and large volume at times, was the presenting symptom in 92% of cases. Other less common presentations included bloody diarrhea, abdominal pain, and steatorrhea. MMF was discontinued or dose reduced in 65%, switched to enteric coated mycophenolate mofetil sodium in 14% and continued in 21% of cases. Ninety-eight percent of cases managed with discontinuation or dose reduction of MMF responded to the treatment. About 93% of cases who switched from MMF to enteric coated mycophenolate mofetil sodium responded. Among the patients who continued MMF, diarrhea was persistent in 17% while it resolved spontaneously in 83% (Figure 1).
The median time to response to either change to enteric coated mycophenolate sodium or discontinuation of treatment was 20 days (range 1-45).
Complications developed in 3.5% (n=19) of cases including graft rejection in solid-organ transplant (n=11) after discontinuation or dose reduction of MMF, acute dehydration (n=6), toxic colitis (n=1), and severe weight loss of >60 pounds (n=1).
Conclusion
MMF-induced colitis generally presents with watery diarrhea but rarely blood may be noted in stool. The latency period can be as long as months to years, hence a long latency period does not exclude the possibility. A vast majority of patients respond to cessation of MMF or dose reduction within a few weeks, however, cessation of MMF may result in graft rejection. In cases where continuation of immunosuppressive therapy is considered important to prevent graft rejection, alternate management option for management of MMF-induced colitis could include switching to a different drug formulation.
Management and outcomes of MMF-induced colitis
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal