Abstract
Introduction: Approximately 18,960 new cases of CLL and 4,660 deaths from CLL are estimated in the US in 2016, with an overall estimated 5-year survival rate of 82%. Despite this, CLL patients with unfavorable genetic features such as 17p deletion have relatively poor outcomes when treated with conventional chemoimmunotherapy (e.g. fludarabine and cyclophosphamide, or bendamustine plus rituximab); however, several new treatments have been approved by the FDA for the treatment of previously untreated CLL in the past 3 years, including obinutuzumab with chlorambucil and ibrutinib. The aim of this analysis was to assess demographics and treatment patterns in patients with previously untreated CLL since the introduction of new treatment options using a novel oncology electronic health record (EHR) database.
Methods: A cohort of CLL patients was selected by identifying patients within Flatiron's real-world oncology database. The Flatiron provider network comprises 230 clinics, 2,000 clinicians, and more than 1 million cancer patients throughout the United States (US). Patients included in the cohort were required to meet the following criteria: ≥2 clinic encounters on different days occurring on or after January 1, 2011; ≥1 medication order for an antineoplastic occurring on or after January 1, 2013; physician documentation of CLL; and, evidence in unstructured documents (ie, information not organized in a pre-existing data model, such as free text from a physician note/lab report) of having been treated specifically for CLL. The latter two criteria were assessed based on technology-enabled abstraction of unstructured data (e.g., pathology reports, clinician notes). Patients who lacked unstructured documents, absence of evidence of first-line treatment, or received CLL treatment at a practice outside of the Flatiron network were excluded. The cohort included patients of all ages treated between 2011 and 2015 from all 50 states of the US.
The index date was defined as the date of the patient's CLL treatment initiation. Start of first-line therapy after January 1, 2011, was defined as the first episode of an eligible therapy given after or up to 14 days before the date of the patient's CLL treatment initiation. Line of therapy was the first eligible drug episode plus other eligible drugs given within 28 days. Therapies eligible for inclusion in lines of therapy were systemic treatment, as evidenced by an order or administration of an antineoplastic agent recorded in the EHR; radiotherapy and surgery were not included.
For patients with documented transformation of CLL, the abstracted date of transformation ended any active line of therapy, and the patient was not considered eligible for any subsequent CLL lines of therapy. Any treatment that occurred after the date of transformation was not included as a CLL line of therapy (steroids were not included in the definition of CLL lines of therapy).
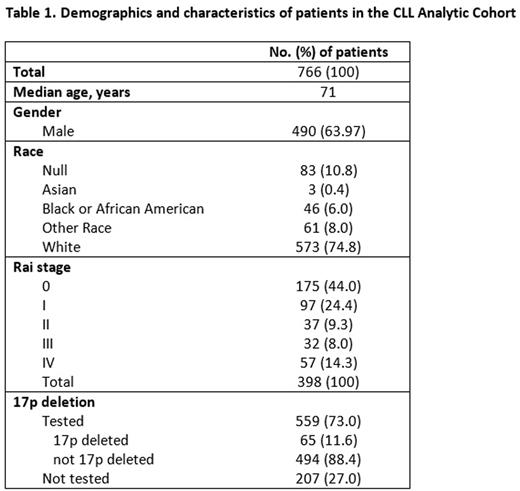
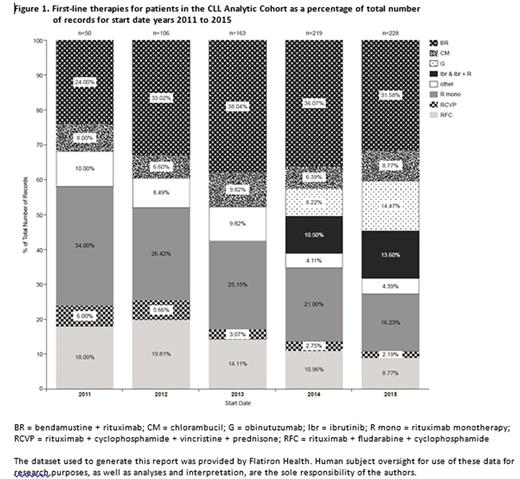
Results: As of June 2016, the cohort consisted of 766 eligible CLL patients with a median age of 71 years, and 64% were male (Table 1). While distribution of first-line therapies initiated in 2011 to 2013 remained relatively constant by year, changes were observed during 2014 and 2015 following the introduction of obinutuzumab and ibrutinib (Figure 1); obinutuzumab monotherapy as first-line therapy increased from 8.2% in 2014 to 14.5% in 2015, and ibrutinib monotherapy or ibrutinib + rituximab increased from 10.5% in 2014 to 13.6% in 2015. Of note, fludarabine containing regimens declined from 19.8% in 2012 to 8.8% in 2015. Decreases were also observed with rituximab monotherapy from 21.0% to 16.2%, bendamustine + rituximab (BR) from 36.1% to 31.6%, and rituximab + fludarabine + cyclophosphamide (RFC) from 11.0% to 8.8%. Factors associated with chlorambucil treatment (as monotherapy or in combination) vs. chemoimmunotherapy included older age (75.9 years vs. 68.7 years and 62.6 years for BR and RFC, respectively) and Rai stage (78.1% of patients treated with chlorambucil had Rai stage 0-I disease vs. 70.1% and 71.7% treated with BR and RFC, respectively). A fifth of patients with 17p deletion were treated with ibrutinib. Updated data inclusive of 2016 treatments will be presented.
Conclusion: Using a novel EHR database, the marked change in CLL treatments from 2011 to 2015 shows increased utilization of newer agents. Further follow-up and analysis will contrast treatment patterns beyond RCT data in a real-world setting.
Arnieri:F. Hoffmann La-Roche Ltd: Employment. Bernaards:F. Hoffmann La-Roche Ltd: Employment. Wilhelm:Roche: Equity Ownership; Genentech: Employment. Black:F. Hoffmann La-Roche Ltd: Employment. Hirst:F. Hoffmann La-Roche Ltd: Employment; AstraZeneca: Other: Previous employment . Taylor:F. Hoffmann La-Roche Ltd: Employment. Lambert:F. Hoffmann La-Roche Ltd: Employment. Green:F. Hoffmann La-Roche Ltd: Employment. Lu:F. Hoffmann La-Roche Ltd: Employment. Humphrey:Genentech, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal