Abstract
Background: Hemophilia-A is a chronic inherited bleeding disorder that results from deficiencies in factor VIII. Prophylactic replacement of coagulation factor VIII (FVIII) is the standard of care. However, about 20-30% of patients with severe hemophilia A may develop neutralizing antibodies (inhibitors) against the coagulation factor VIII. The Immune Tolerance Induction (ITI) method, consist in the regular infusion of variable FVIII doses to induce FVIII antigen-specific tolerance. It has proven the most effective inhibitor eradication treatment in patients with high-titer inhibitors. Current published studies reported wide range of findings on the factor usage and cost of treatment. The objective of this study is to describe the consumption, duration, and cost of ITI treatment in US healthcare settings.
Methods: This retrospective analysis used MarketScan, an US health insurance claim database from January 2010 to September 2015. Eligible patients were: male hemophilia-A inhibitor patients who received ITI treatment. ITI treatment was identified as patients who tested Bethesda/Nijmegen assays between 30 days prior to an ITI index date and the end of study period; and used high dose of factor therapy (>3 times of the median IU factors dispensed for patients in the same age group for more than 5 consecutive months, allowing one month gap). Conclusion of ITI treatment was defined as when the amount of IU dispensed has fallen under 1.5 times of the median amount dispensed, or remaining in ITI treatment at the end of study period. Patients, who used by-pass agents to treat bleeding without undergoing ITI, were excluded. The total cost of ITI treatment was focused only on the cost of factor therapy. The treatment duration, cost, and IU consumption were further stratified by age groups and specific factor therapies.
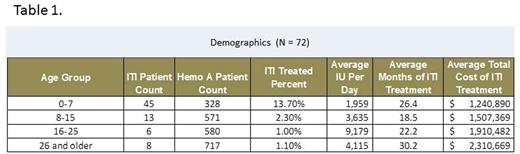
Results: Of the 2,302 hemophilia-A patients, 231 inhibitor patients were identified. A total of 72 patients used high dose short-acting factors for ITI treatment. No patient receiving long-acting factor was captured in the inhibitor population. Table 1 lists the patient counts, duration and cost of ITI treatment by age groups. Higher percentages of younger patient groups developed inhibitor compared with older age groups. About 14% of hemophilia-A patients aged 7 and under had ITI treatment. The older groups (aged 16 and older) had higher total costs as they required higher IU of factor therapy. The average duration of ITI treatment was 18.7 months, and the average total cost was $1,463,668 per patient regardless of the type of short acting factor therapy used.
Conclusions: This US health insurance claim database study reflects that the current utilization of short acting factor for ITI treatment is associated with high cost burden. Products that decrease or eliminate inhibitor formation should be developed.
Su:Novartis Pharmaceuticals Corporation: Employment; Biogen: Employment, Equity Ownership. Zhou:Biogen: Employment, Equity Ownership. Buckley:Biogen: Employment, Equity Ownership. Rising:Biogen: Employment, Equity Ownership. Hou:Biogen: Employment, Equity Ownership. Jain:Biogen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal