Abstract
Background: Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has been used when HLA-matched siblings are not available. Conditioning regimens aim to reduce tumor burden prior to HSCT and provide sufficient immunoablation.
Patients and Interventions: We report the outcome of haplo-HSCT in 63 consecutive patients (19 females/44 males) with high-risk or relapsed/refractory hematological malignancies (n=29-AML; 8-sAML; 19-ALL; 5-advanced-MDS; 2-CML-BC), followed at our Center from 2/2013 to 12/2015. Median age was 20 years (range: 1.1-49). Twenty-one patients achieved remission prior to transplant, while 42 did not (7%-98% BM blasts). No patient had severe infection or organ failure before HSCT. Patients received FA5-BUCY registered on http:// ClinicalTrials.gov (NCT02328950), i.e., 5-day salvage chemotherapy (Fludarabine/Ara-C) and conditioning (Busulfan/Cyclophosphamide). GvHD prophylaxis included ATG, CsA, MMF and short-term MTX.
Results: All patients received stem cells from bone marrow and peripheral blood, and achieved successful engraftment, except two who died before. With a median follow-up of 269 days (120-1081), 42/63 patients are still alive and disease-free. Two-year OS and RFS were similar in patients not in remission and in those in complete remission (61.3% vs 56.3%, p=0.88; 58.3% vs 56.3%, p=0.991). Non-relapse mortality and relapse incidence were 22.2% and 11.1%, respectively. Severe acute-GvHD occurred in 4/63 patients. Transplant-related mortality was low at day+100 (17.5%) and for the entire study period (20.6%). Unexpectedly, few patients experienced mild-to-moderate toxicity, and main causes of death were infection and GvHD. BM blast counts, age,and donor-recipient gender-pairs did not affect the outcome. Less chemotherapy cycles prior to HSCT might result in more favorable outcome.
Conclusions: Thus, haplo-HSCT with FA5-BUCY appears promising for advanced disease, especially when TBI and amsacrine, used for FLAMSA, are not available and in pediatric patients for whom TBI is not recommended.
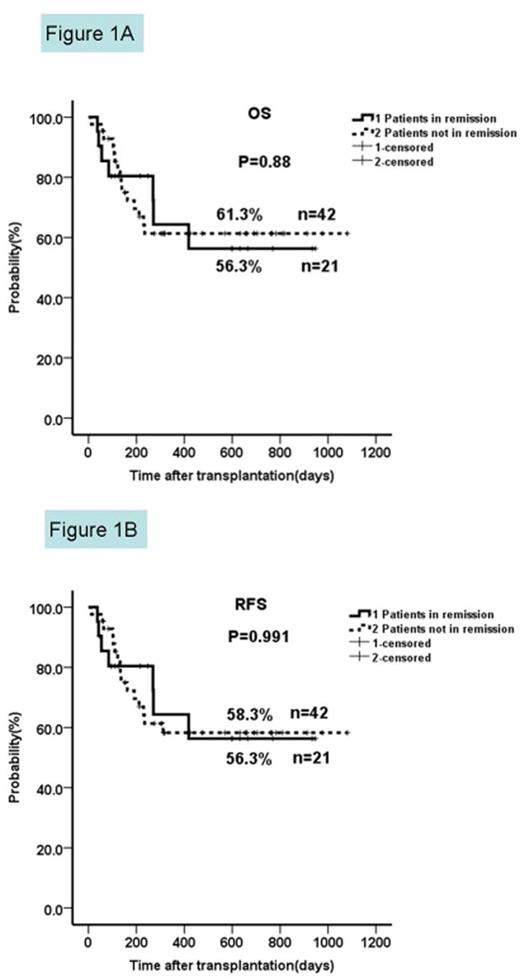
Two-year OS and RFS in patients not in remission prior to HSCT compared to those in complete remission.
The two-year OS and RFS in patients not in remission were similar to those from patients in complete remission (61.3% vs 56.3%, p=0.88; 58.3% vs 56.3%, p=0.991).
Two-year OS and RFS in patients not in remission prior to HSCT compared to those in complete remission.
The two-year OS and RFS in patients not in remission were similar to those from patients in complete remission (61.3% vs 56.3%, p=0.88; 58.3% vs 56.3%, p=0.991).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal