Abstract
Patients with acute lymphoblastic leukemia (ALL) who are refractory to initial and re-induction chemotherapy have a dismal prognosis unless they undergo allogeneic hematopoietic cell transplantation (HCT). Due to a lack of studies investigating primary refractory (PREF) ALL there are no known factors potentially influencing transplantation outcomes in these patients. We believe it is important to identify these factors and clarify outcomes of HCT in PREF ALL in this era of novel therapies for chemo-refractory ALL.
The outcomes of 86 adult patients who underwent their first HCT for PREF ALL between 2000 and 2012 were reported to the European Society for Blood and Marrow Transplantation (EBMT) Registry. PREF disease was defined as failure to achieve complete remission (CR, <5% of blasts in the bone marrow) after two or more courses of induction chemotherapy. A total of 70 patients were transplanted using a myeloablative conditioning regimen, of whom 52 received a total body irradiation (TBI)-based regimen (median dose 12 Gy; range: 8-14). Sixteen patients were transplanted using a reduced-intensity conditioning (RIC) regimen, as defined by the EBMT criteria. In 6 patients, the RIC regimen incorporated TBI at a dose of 6 Gy or less. In vivo T-cell depletion was used in 28 (33%) patients (anti-thymocyte globulin was used in all but 6 who received alemtuzumab). The median duration of follow-up in live patients was 106 months (range 9-181 months). Study main endpoints were survival, CR rate, leukemia free survival (LFS), and non-relapse mortality (NRM).
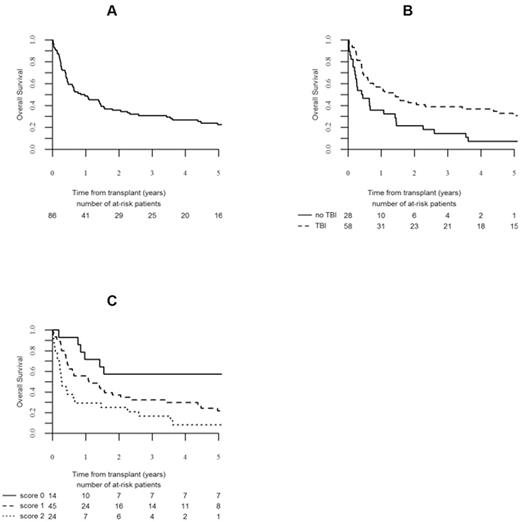
A total of 76 (89%) patients demonstrated neutrophil engraftment at a median of 18 days (9-87) and 9 (11%) patients experienced primary graft failure. Grade II or higher acute GVHD was documented in 28 (34%) patients, while 14 (17%) patients experienced grade III/IV acute GVHD. Chronic GVHD of any severity developed in 27 (32%) patients of those surviving more than 100 days. With a median follow-up of 106 months, 20 patients were alive and free of leukemia. The probability of survival (Figure A) for the whole group was 36% (95% confidence interval (CI) 25 - 46) at 2 years and 23% (95% CI 13 - 32) at 5 years. The probabilities of LFS and NRM at 2 and 5 years were 28% (95% CI 18 - 38) and 17% (95% CI 8 - 25) and 20% (95% CI 12 - 29) and 29% (95% CI 19 - 39), respectively. In all, 66 (76%) patients achieved CR after transplant at a median time of 31 days (28 - 147 days). For patients achieving CR, the survival was 36% (95% CI 25 - 46) and 29% (95% CI 18 - 41) and relapse incidence was 51% (95% CI 38 - 63) and 54% (95% CI 41 - 66) at 2 and 5 years, respectively.
The following factors associated with improved survival and LFS with P <0.10 in univariate analysis entered multivariate analysis: the use of TBI, absence of extramedullary disease, no more than 2 induction courses, male sex, and infusion of female cells into male recipient. In multivariate analysis, use of TBI was associated with improved survival (Hazard Ratio (HR) 0.53; 95% CI 0.29-0.973; P=0.04), (Figure B). Similarly, factors associated with improved LFS were: use of TBI (HR 0.44; 95% CI 0.24-0.82; P=0.01) and infusion of female hematopoietic cells into male recipient (HR 0.45; 95% CI 0.23-0.90; P=0.01). Presence of extramedullary disease was associated with increased NRM (HR 4.92; 95% CI 1.85-13.02; P=0.001). We developed a score using the summation of the number of prognostic factors found to be significant for LFS by multivariate analysis: use of TBI and infusion of female hematopoietic cells into male recipient. This allows delineation of three prognostic groups as follows: score 0 (both prognostic factors present): (N=14), 5 year survival: 57% (95% CI 31 - 83) and 5 year LFS: 50% (95% CI 24 - 76); score 1 (only 1 prognostic factor): (N=45), 5 year survival: 22% (95% CI 9 - 34); and 5 year LFS 12% (95% CI 2 - 22); score 2 (no prognostic factor): (N=24), 5 year survival and LFS: 8 (95% CI 0 - 19) (Figure C).
In conclusion, our data support the use of allogeneic HCT in selected patients with PREF ALL who cannot reach CR. Conditioning regimens should contain TBI and male patients may benefit from a female donor. Clearly there is a need for introduction of modern therapies capable of improving anti-leukemic control prior to and after transplantation.
Stelljes:Pfizer: Consultancy. Fanin:Novartis: Speakers Bureau. Bloor:Janssen: Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Consultancy, Speakers Bureau; Gilead: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal