Background: Modern post-transplant cyclophosphamide (PTCy) protocols with haploidentical (haplo) donors have dramatically expanded the donor pool for patients requiring hematopoietic cell transplantation (HCT). Initial studies were performed with bone marrow grafts, which require the donor to undergo anesthesia during harvest. Consequently, the use of mobilized peripheral blood hematopoietic cells (PBHC) may be desirable, especially with older donors. However, data on PBHC haplo-HCT in older adults is lacking.
Objectives: To report the impact of age on transplant-related outcomes in a large cohort of patients undergoing haplo-HCT with PTCy for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
Methods: We retrospectively identified all adult patients undergoing T cell replete haplo-HCT with PTCy for AML or MDS at our institution from January 2009 to June 2016. Patients were grouped into three cohorts: Age1 (<55), Age2 (55-65) and Age3 (>65). Patients were scored for disease risk and underlying comorbidities using the Disease Risk Index (DRI) and HCT Comorbidity Index (HCT-CI). Overall survival (OS) was analyzed using a Cox Proportional Hazards (CPH) model, with all variables pre-specified.
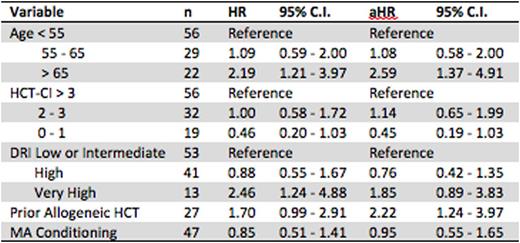
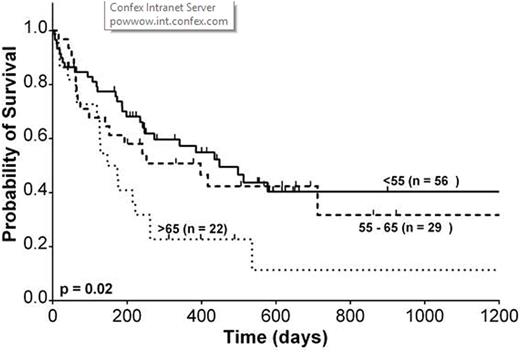
Results: We identified 107 patients, 92 with AML and 15 with MDS. Median follow up was 8.0 months in all patients and 18.3 months in patients surviving at last follow-up. Median donor age was 39, 34 and 49 in Age1, Age2 and Age3, respectively (p = 0.02). Donor relationship was also significantly different among Age1 (child: 25%, sibling: 53%, parent, 22%), Age2 (62%, 38%, 0%) and Age3 (64%, 36%, 0%) (p < 0.001). Younger patients were significantly more likely to receive myeloablative conditioning (55% vs. 35% vs. 27%, p = 0.04). Median OS was 448, 417 and 147 days in Age1, Age2 and Age3 patients. Actuarial 2-year OS was 40%, 34% and 11%, respectively. The 2-year cumulative incidence of relapse was 34%, 30% and 56%. The 2-year cumulative incidence of treatment-related mortality was 30%, 45% and 35%. There was a trend towards a lower 100-day cumulative incidence grade II-IV acute graft-versus host-disease (aGVHD) in older patients (44% vs. 35% vs. 15%, p = 0.07) but not in grade III-IV aGVHD (16% vs. 7% vs. 5%, p = 0.42). Similarly, there was a trend towards a lower 1-year cumulative incidence of cGVHD in older patients (37% vs. 36% vs. 10%, p = 0.07), but not severe cGVHD (5% vs. 0% vs. 0%, p = 0.16). No significant difference was seen in median time to neutrophil engraftment (17 vs. 18 vs. 17 days, p = 0.14) or platelet engraftment (28 vs. 30.5 vs. 32 days, p = 0.93). Univariate and multivariate CPH model of OS is summarized in table 1. Age > 65 and prior allogeneic HCT were associated with significantly worse survival.
Conclusions: The use of PBHC haplo-HCT in older adults with AML or MDS is a feasible treatment option. However, a careful pre-transplant evaluation and analysis of risks and benefits is warranted when offering this transplant modality to older adults. Even after adjusting for pre-transplant comorbidity and disease risk, older age is associated with inferior survival in this cohort.
Overall survival by age
DiPersio:Incyte Corporation: Research Funding. Vij:Takeda: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Honoraria; Celgene: Consultancy; Bristol-Myers Squibb: Honoraria; Janssen: Honoraria; Karyopharm: Honoraria. Schroeder:Incyte Corporation: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal