Abstract
Allogeneic hematopoietic cell transplant (HCT) is potentially curative for myeloid malignancy when indicated. In patients age 60 years or older HCT has become feasible with the use of reduced intensity conditioning (RIC) and studies have shown improved outcome in this older population. There is no clear consensus concerning the superiority of any single reduced intensity conditioning regimen for elderly patients with myeloid malignancies. At the Princess Margaret Cancer Centre we have been using a combination of fludarabine/busulfan plus low dose total body irradiation (TBI) as the sole RIC regimen since the year 2006. We present a retrospective analysis on the outcomes of 116 patients aged 60 to 71 years who underwent allogeneic HCT during the time period 2006-2015 for myeloid malignancies.
A total of 40 patients (34%) underwent RIC HCT during the years 2006-2010 and 76 patients (66%) during the years 2011-2015. Median age at HCT was 64 years (range 60-71). AML was diagnosed in 73 patients (63%) while 43 had other myeloid malignancies (MDS=34, CMML=8, blastic plasmacytoid dendritic cell neoplasm=1). Seventy four patients (64%) had de novo and 42 (36%) had secondary disease. Cytogenetics were available for 108 patients (93%), 17 favorable (16%), 59 intermediate (54%) and 32 unfavorable risk (30%) (MRC criteria for AML and IPSS for MDS/CMML). All leukemia patients were in first remission (<5% blasts) and MDS/CMML patients had less than 10% marrow blasts at HCT. As for pre-transplant comorbidity risk, 59 patients (51%) had HCT-CI score of 0-2 and 57 (49%) had a score of 3 or more. Donors were HLA matched related in 63 patients (54%), all other donors were unrelated including 19 mismatched (16%). Both recipient and donor were cytomegalovirus sero-negative in 28 (24%) cases. Female donor to male recipient occurred in 27 (23%) cases. Grafts were peripheral blood stem cells (PBSC) in almost all the patients (n=114, 98%). All patients (n=116) received RIC conditioning with fludarabine 30mg/m2/day for 4 days (day -5 to day -2), busulfan 3.2mg/kg (adjusted BW)/day for 2 days (day -3 to day -2 and TBI 200 cGy x one dose (on day -1).
Median follow-up of survivors was 39 months (range 6.5 -121). Median neutrophil engraftment (≥0.5 x10e9/L) was 18 days (range 12-35 days), median platelet engraftment (≥20 x10e9/L) was 11 days (range 7-49 days). Two patients never engrafted and 23 patients did not have a decrease in platelets <20 x10e9/L at any time. Acute graft versus host disease (GVHD) grade II-IV occurred in 59 patients (51%) while grade III-IV was seen in 34 patients (29%). Chronic GVHD at any time was seen in 52 patients (45%). Relapse occurred in 27 patients (23%). Of the 81 patients that died, the primary cause of death was GVHD in 25 patients (31%), disease relapse in 25 patients (31%), infection in 18 patients (22%), and graft failure in 6 patients (7%). Data was not available in 3 patients (4%) and 4 patients (5%) died from other causes.
Overall survival (OS) for the entire cohort at 3 years was 33% (95% CI 24-42) on univariate analysis. Type of myeloid malignancy (AML versus others, p=0.007), donor type (p= 0.001), HLA mismatch (p= 0.06) and transplant time period (p= 0.05) were the only variables which showed p<0.1 on univariate analysis for OS. Cumulative incidence of relapse (CIR) at 3 years was 24% (95% CI 16-32). Non-relapse mortality (NRM) at 3 years was 43% (95% CI 34-52). Multivariable analysis for OS using the variables with p<0.1 demonstrated AML patients to have a superior outcome compared to other myeloid malignancies (HR 0.62, 95% CI 0.39-0.99, p=0.045), as well as patients with related donors (HR 0.52, 95% CI 0.33-0.82, p=0.005). For CIR none of the variables were found to be significant. For NRM, AML patients had superior outcome (HR 0.57, 95% CI 0.33-0.97, p=0.038), as well as patients with related donors (HR 0.55, 95% CI 0.33-0.94, p=0.028).
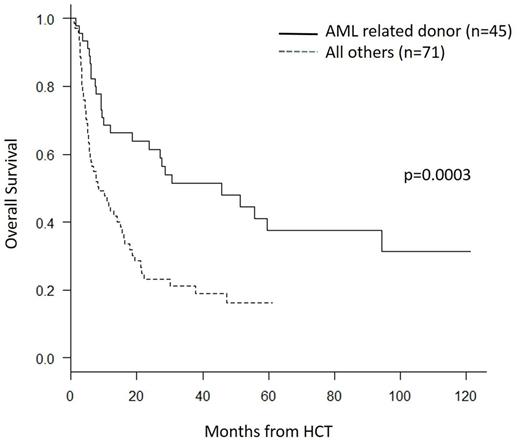
Based on the above findings, OS was determined for the AML patients that underwent related donor transplant with this regimen (n=45). Three year OS was 51% (95% CI 36-65) for the AML patients who underwent related donor transplant versus 21% (95% CI 12-32) for the group which included AML with unrelated donors and those with other myeloid malignancies (p= 0.0003) (see figure). We conclude that fludarabine/busulfan plus low dose TBI is an effective conditioning regimen for older patients with myeloid malignancies, in particular for AML patients in first remission with matched related donors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal