Abstract
Background: The prognosis of multiple myeloma (MM) has improved significantly over the last 20 years due to the introduction of autologous stem cell transplantation and novel drugs. The standard regimen for conditioning in MM is melphalan 200 mg/m2, since a study published in 2002 (Moreau et al.) showed a survival advantage of melphalan (MEL) only over melphalan- total body irradiation (MEL-TBI) conditioning. Ionizing radiation is an independent treatment modality and has activity in MM. We decided to reevaluate the outcome of MEL-TBI conditioning in the age of novel drugs.
Patients and Methods: In a retrospective chart review, we identified 50 patients with MM who underwent autologous hematopoietic cell transplantation at Tulane University Medical Center and were conditioned with MEL-TBI between December 1995 and March 2012. Stem cells were collected following a stimulation by 10 µg/ kg filgrastim for 4 days. In case of insufficient collection, plerixafor was administered. Between 1995 and 2003 cyclophosphamide priming was used in most cases. Patients received melphalan 140 mg/m2 as a single dose given intravenously on day -5. TBI was administered in fractionated doses of 150 cGy between days -4 and -1 (total dose 12 Gy). Peripherally harvested stem cells were infused on day 0. Standard supportive measures were followed. Log-rank test and Kaplan-Meier curves were used to calculate overall survival (OS) and progression-free survival. Significance levels were standard (p<0.05). The expected survival for each patient was compared with data from the Louisiana Tumor Registry (matched with 5 year age interval and 4 year time of diagnosis interval).
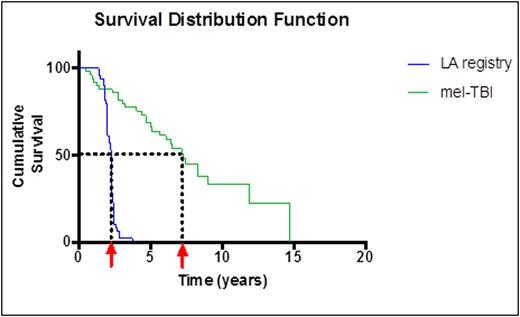
Results The OS from the date of diagnosis is shown in Fig. 1 (84.2 months). The mean survival from date of transplant to death or last follow-up is 70.1 months. No significant differences in OS were observed according to gender, race, age at transplant, or initial stage of disease. No significant differences were observed when patients transplanted in remission (CR, sCR or VGPR) were compared with patients with partial remission, stable or progressive disease. Patients treated more recently (2006-2013) had a trend to a longer survival compared with the earlier period (1995-2005). Seven out of 8 patients who were not in CR achieved CR post-transplant. The d100 survival in the earlier period was 83%, in the later period 97%. The 1 year survival increased from 72% in the earlier period to 91% in the later period. When the survival of patients transplanted at our center is compared with the observed survival of all MM patients in Louisiana, a dramatic difference becomes apparent (median survival 27 months versus 84 months, see Figure 1). Second malignancies were documented in 3 (6%) of our patients (T-cell lymphoma, renal cell carcinoma, possible myelodysplastic syndrome).
Conclusion: Mel-TBI conditioning results in long-term survival, especially if the early toxicity associated with total body irradiation is ameliorated. The survival reported in this study is almost twice as reported by Moreau et al. The improvement is in large part due to salvage treatments and improved supportive care. However, MEL-TBI may be superior in certain subgroups of patients. We plan to perform a larger study comparing MEL-TBI to MEL only with risk stratification and to investigate the possible interaction between cyclophosphamide priming and early toxicity. According to our data, MEL-TBI does not result in increased second malignancies. The survival of patients who underwent transplant with MEL-TBI is superior to patients in a population-based registry (matched for age and time of treatment).
Kaplan-Meier survival distribution of patients treated by autologous transplantation with MEL-TBI (green curve) compared with registry data (blue curve) from time of diagnosis to last follow-up or death
Kaplan-Meier survival distribution of patients treated by autologous transplantation with MEL-TBI (green curve) compared with registry data (blue curve) from time of diagnosis to last follow-up or death
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal