Abstract
Abstract
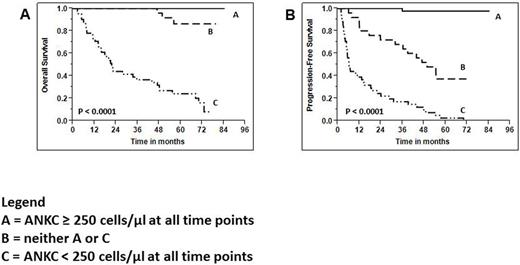
Our group recently reported a Phase III clinical trial (Porrata et al. Biology of Blood and Marrow Transplantation 2016; 22: 1017-1023) demonstrating that the infusion of the autograft-absolute lymphocyte count (A-ALC) and autograft natural killer cells (A-NKC) are survival prognostic factors in non-Hodgkin lymphoma (NHL) patients treated with autologous peripheral blood hematopoietic stem cell transplantation (APBHSCT). A limitation of our Phase III clinical trial was the short-term follow-up of just two years. Therefore, we evaluated longer follow-up and assessed the role of the infused A-ALC, infused A-NKC, and peripheral blood absolute natural killer cell count (ANKC) recovery on survival post-APBHSCT. Of the 122 patients that participated in the Phase III trial, 111 patients were able to complete APBHSCT and included in the current study. With a median follow-up of 57.2 months (range: 2.1-84.6 months), superior overall survival (OS) and progression-free survival (PFS) was observed in patients infused with an A-ALC ≥ 0.5 x 109 lymphocytes/kg [OS: HR = 0.429, 95%CI, 0.128-0.828, p < 0.01; and PFS: HR = 0.456, 95% CI, 0.177-0.863, p < 0.01] and an A-NKC ≥ 0.09 x 109 cells/kg [OS: HR = 1.58e-3, 95% CI, 1.046e-6-0.179, p < 0.003; and PFS: HR= 1.58e-3, 95% CI, 7.81e-7-0.064, p < 0.003]. Furthermore, patients that maintained an ANKC ≥ 250 cells/µl at 3 months, 6 months, 9 months and 12 months post-APBHSCT also experienced superior OS (Figure 1A) and PFS (Figure 1B)[OS and PFS times in months were evaluated from infusion date of stem cells]. In the multivariate analysis a sustained ANKC ≥ 250 cells/µl was an independent predictor for OS (HR = 0.013, 95% CI, 0.001-0.063, p < 0.0001) and PFS (HR = 0.014, 95% CI, 0.001-0.067, p < 0.0001). This study is an extension of our Phase III clinical trial showing that sustained innate immunity by measuring the ANKC correlated with superior clinical outcomes; thus providing a platform to develop innate immunity immunotherapeutic strategists directing to improve clinical outcomes post-APBHSCT in NHL patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal