Abstract
Introduction: For patients with relapsed or refractory DLBCL with chemosensitive disease after 2nd line (salvage treatment), AutoHCT is considered the standard of care. Risk factors for progression following AutoHCT include primary refractory disease and early relapse (<12 months after initial chemotherapy. In our institution, we have previously shown that a course of ICT given prior to AutoHCT in patients with these high-risk features results in improved outcomes in DLBCL and other lymphomas (Damon et al, BMT 2008; 42(10):649-57). More recently, cell of origin (COO) subtype and double myc and bcl2 protein expression (DPP) status have been shown to predict outcome to front line treatment but data in the relapsed setting are lacking. We hypothesized that our approach of ICT followed by AutoHCT will increase the likelihood of complete remission (especially in patients with high-risk clinical or biologic features) and improve progression free survival (PFS) following AutoHCT.
Methods: Consecutive patients who underwent ICT followed by autoHCT for relapsed/refractory DLBCL at our institution between 2005-2015 and had available tissue samples for review were included in our analysis. ICT was applied per institutional standard to patients with apriori identified high-risk features as described below. This chemotherapy step is utilized for stem cell mobilization following evidence of chemosensitivity and prior to undergoing AutoHCT. COO was determined using the Hans algorithm and DPP status using the Johnson criteria. Following CHR approval, patient's charts and EMR were reviewed to ascertain baseline variables and outcome data. Response was determined using radiologic (CT) and metabolic (PET) imaging for all patients at the time of transplantation. Survival probability was estimated by the Kaplan-Meier method. Comparisons were made using the X2 test and Fisher's exact test, where appropriate.
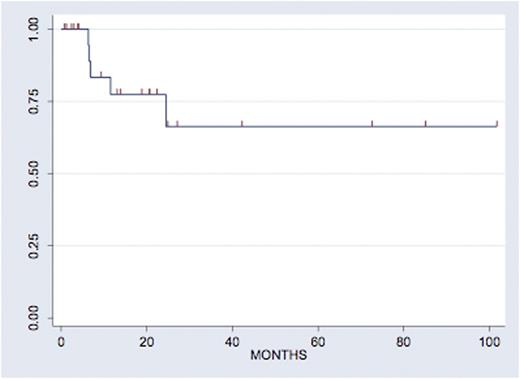
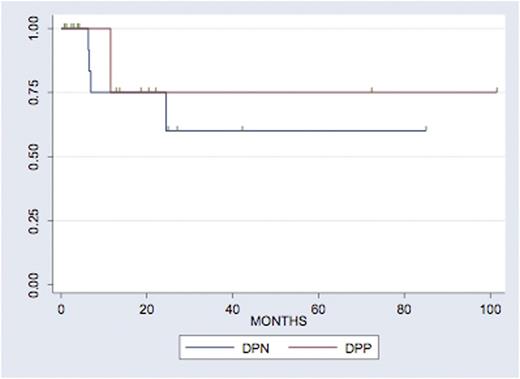
Results: A total of 35 patients were included in this analysis. Out of those, 30 patients received a high-dose etoposide and ara-C-based ICT regimen as described by Damon et al. 13/30 (43.3%) were female, 17/30 (56.7%) were male. Median age at transplant was 56 (range 34-68). The high risk features that lead to this treatment were: early (<12 months) relapse (9/30, 30%) or refractory disease (13/30, 43.3%)to front line therapy or other (8/30, 27.7%). In all, 16/30 (53%) patients achieved CR at the end of salvage therapy, with this proportion increasing to 26/30 (86.7%) CR after ICT. Eventually, 24/30 (80%) of patients were able to continue on to AutoHCT, 23/30 (76.7%) with CBV, 4/30 (13.3%) with BEAM, and 3/10 (10.0%) other. With a median follow up of 20 months, 66% of patients are progression-free at 24 months (Figure 1). In our cohort, 8/30 (27%) were germinal center B-cell origin subtype (GCB), 19/30 (63.3%) were non-GCB subtype and 3 (10.0%) could not be identified. In all, 8/30 (26.7%) were double protein positive, 18 (60.0% were double protein negative and 4 (13.3%) could not be determined. Notably, 15.8% of patients with non-GCB were double protein expressors, compared to 37.5% of patients with GCB (Fisher's exact p=0.06). Neither COO nor DPP were associated with response rates prior to AutoHCT (p=0.6 and p=0.17 respectively) or with PFS (p=0.32 and p=0.72 respectively and Figures 2a and 2b).
Conclusions: Even though COO and DPP status are associated with inferior outcomes in the front-line setting of patients with DLBCL, data in the relapsed setting are limited. In a cohort of high-risk patients with relapsed DLBCL, we confirmed our previously described excellent outcomes with ICT in terms of increased CR rates prior to AutoHCT and a high rate of 2-year PFS. Moreover, while numbers are small, our study suggests that ICT leads to similar outcomes regardless of cell of origin or double protein expression status.
Martin:Amgen: Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal