Abstract
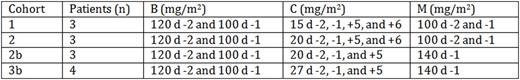
High-dose melphalan (M) followed by autologous hematopoietic stem cell transplantation (AHSCT) remains the standard treatment for multiple myeloma in eligible patients, even in the era of novel agents. However, the majority of patients ultimately relapse and succumb to their disease. Several studies have explored integrating novel agents into the conditioning regimen prior to AHSCT in order to improve complete remission rates and ultimately overall survival. We aimed to assess the feasibility of adding bendamustine (B) and carfilzomib (C) to melphalan (BCM) and performed a phase I dose escalation study. Thirteen patients were enrolled between June 2014 and June 2016. All patients received C at a fixed dose (20 mg/m2) on days (d) -29, -28, -22, -21, -15, -14. The conditioning regimen and doses administered for each cohort were as described in the table below. Due to excessive toxicity, the study was amended after the first 6 patients. Per oversight of a data safety monitoring board, the dose of M was reduced to 140 mg/m2 and C dose on d +6 was omitted. Median age was 58 years (39-68). There were 8 males and 5 females. Performance status was ≥ 80% in all patients. Per the International Staging System (ISS), 3 patients had stage I disease, 5 had stage II, 4 had stage III, and 1 had unknown staging. Three patients had high-risk cytogenetics: 2 with t(4;14) and 1 with deletion 17p. Three patients had received prior AHSCT. Disease status prior to study treatment was stable disease (SD) (n=2), partial response (PR) (n=8), or very good partial response (VGPR) (n=3). Median CD34+ cell dose was 3.24x106/kg (2.23-6.92x106). Median follow-up was 14.2 months (1-24.5). Median time to neutrophil engraftment was 12 d (11-15). One patient died before achieving platelet engraftment. For the remaining patients, median time to platelet engraftment was 16 d (12-20). Non-hematologic toxicities included grade 3 acute mucositis (n=1), lower GI complications (n=6), electrolyte disturbances (n=6), transaminase elevation (n=1) renal insufficiency (n=1), pulmonary edema (n=1), prolonged QTc (n=1), atrial fibrillation (n=1), and elevated troponin (n=1) and grade 4 acute sepsis (n=2), resulting in 1 death in cohort 2 on d +44. Seven patients went on to receive maintenance therapy: 3 with bortezomib, 3 with lenalidomide, and 1 with lenalidomide, dexamethasone, and C. Post-transplant disease status was assessed per protocol by SPEP, SPIF, and serum free light chains and light chain ratio. Nine patients were evaluable on d +100. One patient had SD, 6 had VGPR, and 2 had complete response (CR). Six out of 7 (86%) evaluable patients on d +365 remain disease progression-free.
In summary, BCM conditioning prior to AHSCT at the doses described in cohort 3b seems feasible with manageable toxicities. Five additional patients are being enrolled at the same dose level.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal