Abstract
Introduction:
Hematologic response (HR) particularly complete response (CR) is critical in patients (pts) with AL since it improves overall survival (OS) because of organ response (OrgR). Bor based therapies especially in combination with cyclophosphamide and dexamethasone (CyBorD) have shown to be an active regimen in AL. So far, there have been no comparative studies studying the effect of treatment with B-based regimens prior to SCT (Bor-SCT) versus SCT upfront or SCT preceded by non-Bor therapies (non-Bor-SCT). We present a retrospective analysis of data with an aim to compare the OS, OrgR and HR in the two cohorts.
Methods:
Charts were reviewed for the pts who underwent SCT for AL since 2004 until 2015 at Mayo Clinic in Arizona. HR and OrgR were determined using the consensus criteria from International Society of Amyloidosis.1 HR were noted at 3, 6 and 12 months from SCT.
Results:
Patients and disease characteristics: 63 pts were identified who underwent SCT for AL, of which 34 were in Bor-SCT and 29 were in the non-Bor-SCT cohort. Median age at diagnosis was 59.5 (range, 39 - 72) and 63 (range, 38 - 76) years and at SCT was 60 (range, 40 - 72) and 63 (range, 39 - 76) years for Bor-SCT and non-Bor-SCT, respectively. There were 13 (38%) females in Bor-SCT and 9 (31%) in non-Bor-SCT. Mean bone marrow involvement of plasma cells at diagnosis was 18% (SD±16.5) in Bor-SCT and 6.8% (SD±7.8) in non-Bor-SCT (p = 0.004). All pts had an ECOG performance score of better than 3 at the time of SCT. Fifteen (44%) pts had single organ involvement at diagnosis and 9 (26.5%) had 2-organ involvement in Bor-SCT. In non-Bor-SCT, 21 (72%) pts had single and 6 (21%) had 2-organ involvement (p = 0.04). More than 2 organs were involved in 10 (29%) pts in Bor-SCT and 2 (7%) in non-Bor-SCT. Heart was involved in 18 (53%) pts in Bor-SCT and 8 (28%) in non-Bor-SCT (p = 0.04) while kidney involvement was seen in 27 (79%) in Bor-SCT and 23 (79%) in non-Bor-SCT (p = 0.99). Median creatinine was not significantly different in the 2 groups at diagnosis or at the time of SCT. In Bor-SCT, no pts were on hemodialysis at the time of diagnosis or SCT. In non-Bor-SCT, 1 pt at diagnosis and 3 pts at SCT progressed to need hemodialysis. Lambda light chain was involved in 27 (82%) and 22 (76%) (p = 0.56) with a mean level of involved light chains being 59(SD±88.7) g/dL and 43.3 (SD±64.6) g/dL (p=0.4539) in Bor-SCT and non-Bor-SCT, respectively.
Treatment: Thirty-one (91%) out of 34 pts in Bor-SCT group got treatment with CyBorD while 2 (6%) got Bor with dexamethasone and 1 (3%) got Bor with pegylated liposomal doxorubicin. In the non-Bor-SCT group, 20 (69%) out of 29 got no treatment, 4 (14%) got melphalan prednisone, 2 (7%) got dexamethasone alone, 1 (3%) got vincristine dexamethasone and 2 (7%) got lenalidomide dexamethasone prior to SCT.
Transplant factors: Median time from diagnosis to SCT was 226.5 (range, 65 - 1108) days in Bor-SCT and 150.5 (48 - 1139) days in non-Bor-SCT (p=0.32). Full dose of melphalan (200 mg/m2) was used in 21 (62%) pts in Bor-SCT and 13 (34%) pts in non-Bor-SCT (p <0.001). Mean dose of cells used for SCT was 4.6 (SD±0.9) and 4.8 (SD±2.2) x 106/kg in Bor-SCT and non-Bor-SCT, respectively. Median time to neutrophil and platelet engraftment was 13 (range, 10 - 27) and 16 (range, 8 - 28) days in Bor-SCT while 11 (range, 7 - 16) and 13 (range, 10 - 25) days in non-Bor-SCT.
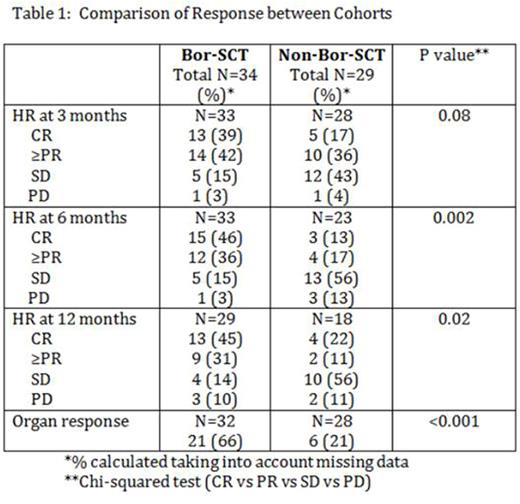
Responses: HR at 3, 6 and 12 months from SCT and OrgR by the time of last follow up is described in table 1.
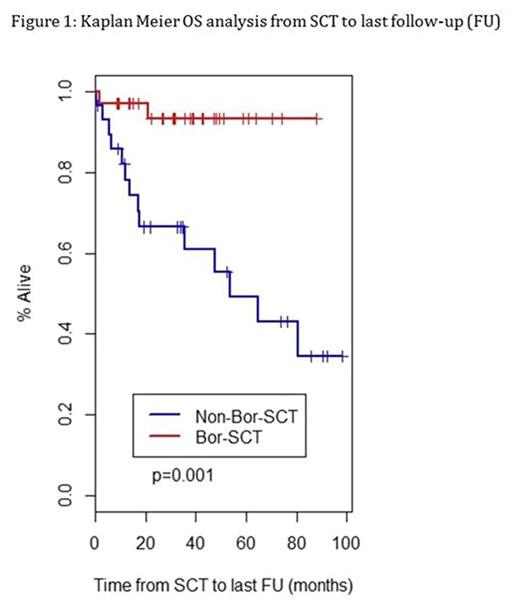
Survival: Median OS was not reached in Bor-SCT as compared to 53.4 months in non-Bor-SCT (p =0.001), Figure 1.
Conclusions:
Our study suggests a benefit in HR, OrgR as well as OS by reduction of disease burden by Bor-based therapy prior to SCT. This benefit was observed despite a higher percentage of pts with cardiac involvement, more than 2 organ involvement and mean bone marrow plasma cells at diagnosis in Bor-SCT. Currently, it is not standard practice to use Bor-based therapy prior to SCT. A prospective study with larger number of pts would be required to confirm these findings.
References:
1. Gertz MA et al. Definition of Organ Involvement and Treatment Response in Immunoglobulin Light Chain Amyloidosis (AL): A Consensus Opinion From the 10th International Symposium on Amyloid and Amyloidosis. Am J Hematol. 2005;79(4):319-328.
Dueck:Bayer: Honoraria. Mikhael:Abbvie, Celgene, Onyx, Sanofi: Research Funding. Bergsagel:Amgen, BMS, Novartis, Incyte: Consultancy; Novartis: Research Funding. Reeder:Novartis, Millennium, BMS and Celgene: Research Funding. Stewart:celgene: Consultancy. Fonseca:AMGEN: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Sanofi: Consultancy; Bayer: Consultancy; Millennium, a Takeda Company: Consultancy; Millennium, a Takeda Company: Consultancy; AMGEN: Consultancy; BMS: Consultancy; Celgene: Consultancy; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; AMGEN: Consultancy; AMGEN: Consultancy; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; Millennium, a Takeda Company: Consultancy; Sanofi: Consultancy; Janssen: Consultancy; Millennium, a Takeda Company: Consultancy; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; Celgene: Consultancy; BMS: Consultancy; Bayer: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal