Abstract
Introduction
High dose chemotherapy and autologous stem cell transplantation (ASCT) is an effective therapy for eligible patients with newly diagnosed systemic AL amyloidosis. Dose attenuated melphalan is used in patients with older age, renal dysfunction, and multi-organ involvement in an effort to limit treatment related mortality. Reducing the dose of melphalan compromises response rates and survival outcomes, but this is often contributed to frailty of these patients. The revised Mayo staging (MS) system improves risk stratification and helps develop risk-adapted therapies. We analyzed the outcomes of ASCT following standard [(200 mg/m2); SD group] versus risk adapted [(140-160 mg/m2); RD group] melphalan conditioning, in the context of the factors typically leading to dose modification.
Methods
Three hundred and twenty nine patients with newly diagnosed systemic AL amyloidosis who underwent conditioning with melphalan followed by ASCT at Mayo Clinic between 2006 and 2015 were identified using existing databases. The clinical and laboratory data, treatment and follow - up details were collected by chart review and analyzed retrospectively. Overall survival(OS) was defined as the time from start of initial therapy until death from any cause or last follow up. Progression free survival(PFS) was defined as the time to relapse or progression requiring change or reinstitution of treatment or death from any cause. The OS and PFS curves were estimated using Kaplan-Meier method while log rank test was used to estimate their difference. Multivariate survival analysis was performed using the Cox proportional hazards model.
Results
Among the 329 patients, 240 patients were in SD group and 89 patients were in RD group (1 patient received 120 mg/ m2, 2 received 160 mg/m2 and the rest received 140 mg / m2).
In the SD group, median age was 58.5 years (25.6 - 72.3); 59.6% were males; 38.7% / 61.7 %/ 7.5% had cardiac / renal /hepatic involvement; and 47.1% / 35.7% / 8.8% / 8.4% had MS I/II/III/IV. In the RD group, median age was 60.8 years (40 - 73.7); 62.9% were males; 66.3% / 71.9 %/ 11.2% had cardiac / renal /hepatic involvement; and 26.2% / 32.5% / 21.2% / 20% had MS I/II/III/IV.
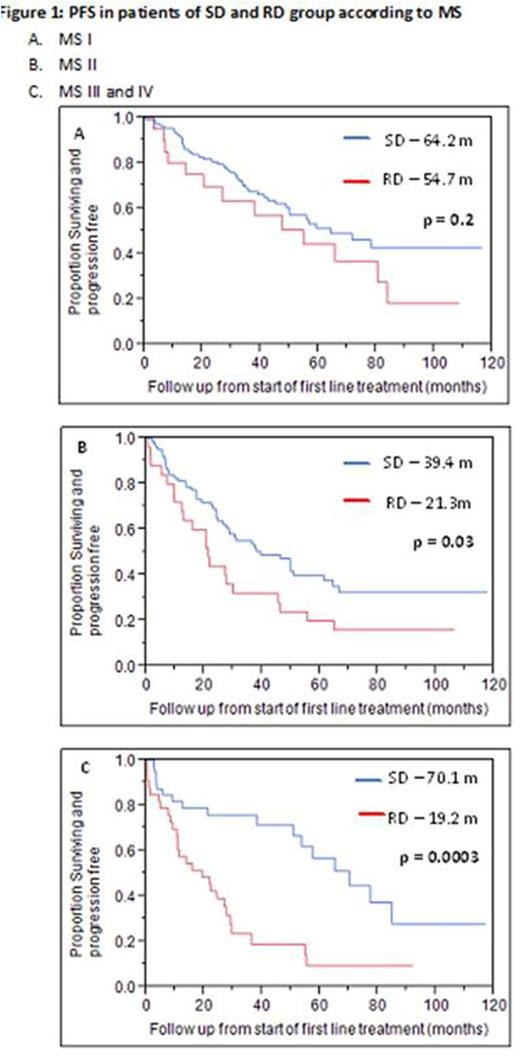
The response rates for patients in the SD group [complete response (CR) - 53.7%, very good partial response (VGPR) - 19.6%, partial response (PR) - 12.5%, no response (NR) - 1.2%, progressive disease (PD) - 1.6%] were significantly higher than patients in the RD group (CR - 34.8%, VGPR - 20.2%, PR - 15.7%, NR - 10.1%, PD - 1.1%) (85.8 vs 70.7%; p < 0.002). The PFS and OS for both the groups according to Mayo stage are presented in Figure 1.
Multivariate survival analysis for PFS and OS was performed using age (≤ vs > 70 years), serum creatinine (≤ vs > 2 mg/dL), number of major organs involved (heart, kidney, liver, gastrointestinal tract and autonomic neuropathy), MS (1 vs 2 vs 3 and 4) and dose of melphalan (SD vs RD). The dose of melphalan (p=0.0002), MS (p=0.03) and the number of organs involved (p=0.04) were significant for PFS while the dose of melphalan (p=0.0001) and the MS (p = 0.004) were significant for OS.
Conclusion
Reducing the dose of melphalan during conditioning for frontline ASCT in systemic AL amyloidosis renders more patients eligible for transplant but compromises on the response rates. There is no impact on PFS and OS in Mayo stage I patients. However the OS as well as PFS in Mayo stage II, III and IV were significantly lower in RD group as compared to SD group. These results suggest a suboptimal outcome with use of reduced dose melphalan, even after accounting for the pother prognostic factors, suggesting that high dose therapy should potentially be limited to those eligible to receive the full dose conditioning.
Dispenzieri:Jannsen: Research Funding; pfizer: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Alnylam: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kapoor:Takeda: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Kumar:Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Research Funding; BMS: Consultancy; AbbVie: Research Funding; Array BioPharma: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Onyx: Consultancy, Research Funding; Glycomimetics: Consultancy; Kesios: Consultancy; Noxxon Pharma: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Millennium: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal