Abstract
Introduction: Despite the introduction of highly effective novel agents, the outcome of patients with relapsed and refractory multiple myeloma (RRMM) remains poor, particularly in those with disease refractory to both proteasome inhibitors (PI) and immunomodulatory agents (IMiDs). Limited available data suggests that autologous hematopoietic stem cell transplantation (auto-HCT) may be an effective therapy in this patient population.
Methods: We retrospectively analyzed all patients with RRMM who underwent first auto-HCT at our center between March 2000 and October 2015. RRMM was defined as never achieving a response (stable disease [SD]) or having progressed while on therapy or within 60 days after discontinuation of therapy. Patients with disease refractory to at least one PI and at least one IMiD either in combination or administered separately were classified as double refractory (DR-MM).
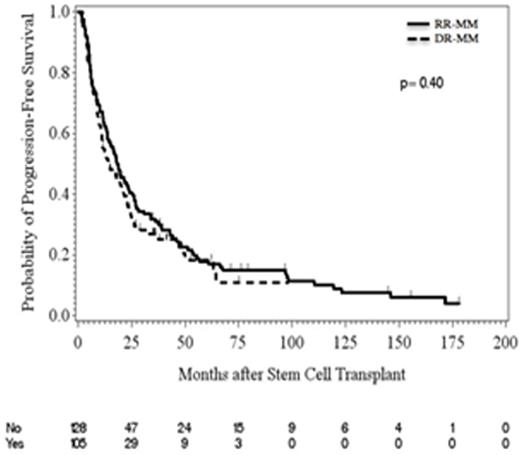
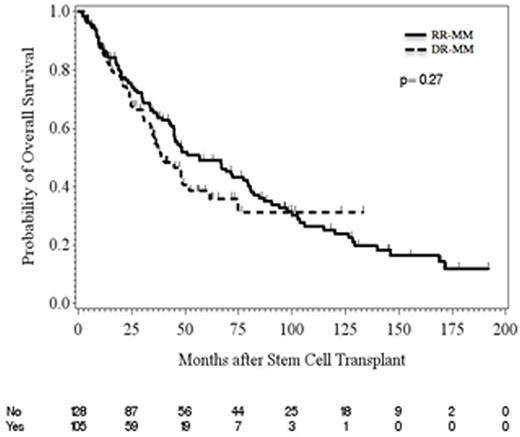
Results: 233 patients with RRMM were identified. Of these, 105 (45%) had DR-MM. The remaining 128 (55%) patients were classified as refractory (R)-MM and included all patients with RRMM but without history of being double refractory. Median age at auto-HCT was 59 years (DR-MM 60 vs. 56 years in R-MM, p=0.005). High-risk cytogenetics (IMWG criteria) were noticed in 67 of 140 (48%) patients (DR-MM, 35/89 [39%] vs. R-MM, 32/51 [63%], p=0.009). Median number of prior lines of therapy was 2 (range 1 - 7) (DR-MM, 2 (1 - 7) vs. R-MM, 1 (1 - 5), p<0.001). Eighty-two (35%) patients received induction with triplet chemotherapy regimens (DR-MM, n=55 [52%] vs. R-MM, n=27 [21%], p<0.001). Chemomobilization was used in 94 (40%) patients (DR-MM, n=54 [51%] vs. R-MM, n=40 [31%], p=0.002). Median time from diagnosis to auto-HCT was 9.4 months (DR-MM 12 vs. 8 months in R-MM, p<0.001). Conditioning regimen consisted of melphalan alone in 168 (72%) and various combinations with melphalan in 65 (28%) patients with no significant difference between DR-MM and R-MM. Maintenance therapy was used in 113 (49%) patients (DR-MM, n=63 [60%] vs. R-MM, n=50 [39%], p=0.001). With a median follow up of 36 months post auto-HCT, at least partial response was seen in 188 (81%) patients (DR-MM, n=83 [79%]; R-MM, n=105 [82%], p=0.50). Near complete remission or better was seen in 52 (22%) patients (DR-MM, n=25 (24%); R-MM, n=27 (21%), p=0.64). The cumulative incidence of non-relapse mortality (NRM) at day 100 and 6 months was 1% and 2% (DR-MM, 0% and 1%; R-MM 2% each at day 100 and 6 months, p=0.56), respectively. The median progression-free survival (PFS) was 17.6 months (14.4 months in the DR-MM and 18.2 months in the R-MM, p=0.40) (Figure 1). Median overall survival (OS) was 48 months (39 months in DR-MM and 57 months in R-MM, p=0.27) (Figure 2). When accounting for other significant measures (i.e., hemoglobin level and β2 microglobulin), having high-risk chromosomal abnormalities was significantly associated with worse PFS (p=0.006) while worsening of PFS for patients with ISS stage II or III disease approached significance (p=0.06). A significant association between OS and hemoglobin level, β2 microglobulin, high risk cytogenetics, ISS stage, and induction treatment was observed, however, none of these measures remained significant in the multivariable model.
Conclusions: Our findings highlight that auto-HCT is an effective and safe therapy in patients with RRMM including those who are refractory to an IMiD and PI
Ciurea:Cyto-Sen Therapeutics: Equity Ownership; Spectrum Pharmaceuticals: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal