Abstract
Recipients of T-cell depleted allogeneic hematopoietic stem cell transplants (SCT) are at high risk forEpstein Barr Virus (EBV) reactivation and post-transplant lymphoproliferative disease (PTLD). Early treatment strategies, including chemotherapy and de-escalation of immunosuppression, proved unsuccessful. Unselected donor leukocyte infusions (DLIs) can be effective, but increase the risk of graft-versus-host disease (GVHD). Rituximab is increasingly used as preemptive therapy for EBV viremia to prevent PTLD development, leading to improved outcomes.However, there are limited data on rituximab-related toxicities in this population. The purpose of this study is to retrospectively describe these outcomes and toxicities in CD34+ selected SCT.
Patients who received a CD34+ selected SCT between January 2008 and February 2015 at Memorial Sloan Kettering Cancer Center were evaluated for inclusion. All patients with EBV reactivation post-transplant were included. Proven PTLD was defined as biopsy-confirmed, with corresponding clinical manifestations. Probable PTLD was defined as significant lymphadenopathy or end-organ disease with EBV viremia in the absence of another cause. All patients with EBV reactivation received rituximab per institutional guidelines.
Response to rituximab was assessed by viral clearance and the criteria for PTLD, described by Cheson, et al. Patients without repeat imaging were assessed for clinical response (resolution of PTLD signs and symptoms without need for additional therapy). Toxicities assessed 1 year post-rituximab include new-onset hypogammaglobulinemia (IgG <500 mg/dL), new-onset neutropenia, (absolute neutrophil count ≤1000/mm3), and infections (e.g. viral, bacterial, and fungal).
The cumulative incidence of each outcome was determined using the cumulative incidence method for competing risks, with death as the competing event. A landmark analysis investigated possible differences in outcomes related to the number of rituximab doses received by 4 weeks (>3 or ≤3 doses). Differences in outcomes due to EBV diagnosis, prior GVHD or immunosuppressant use, or number of rituximab doses by 4 weeks were evaluated using Gray's test. For all analyses, pvalues <0.05 were considered statistically significant.
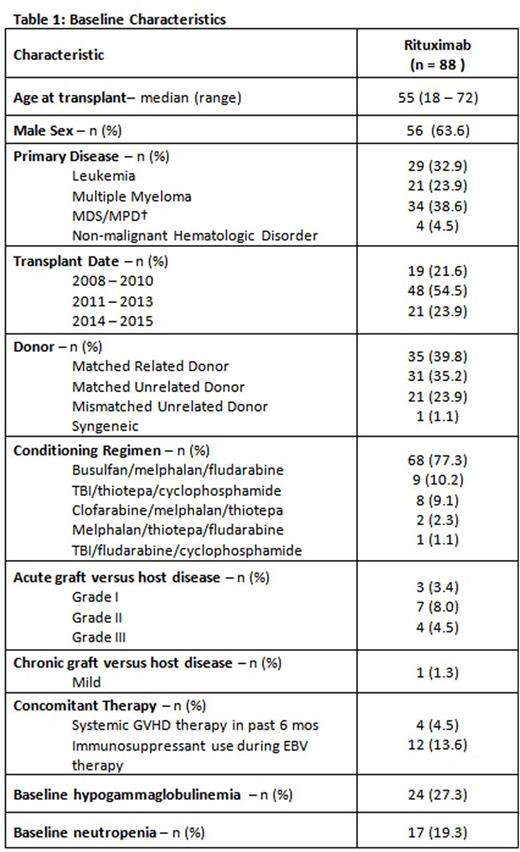
Eighty-eight patients had EBV reactivation (16.6% of CD34+ selected SCT recipients). EBV viremia was most common (n=42), followed by probable PTLD (n=28), and proven PTLD (n=18). Baseline demographics are summarized in Table 1.
Patients with EBV viremia received fewer doses of rituximab (median 2, range 1 - 5) compared to patients with probable (median 4, range 2 - 5) or proven PTLD (median 4, range 3 - 11). Viral clearance occurred in >95% of patients with EBV viremia and probable PTLD versus 77.7% of patients with proven PTLD. For EBV viremia, viral clearance occurred after a median of 1 dose of rituximab (range 1 - 4).
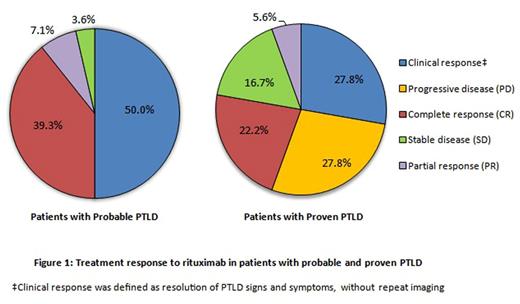
Figure 1 depicts treatment responses to rituximab. The complete response (CR) rate was higher in patients with probable versus proven PTLD (39.3% vs. 22.2%). Patients with probable PTLD also had a higher clinical response rate (50.0% vs. 27.8%). Only patients with proven PTLD received subsequent therapy after rituximab (45.5%).
The cumulative incidence of infection of any type was 71.8% (95% confidence interval [CI], 60.9 to 80.1) at 1 year post-rituximab. By univariate analysis, infectious outcomes were not impacted by number of rituximab doses by 4 weeks, EBV diagnosis, prior GVHD or immunosuppressant use. New-onset neutropenia occurred in 39/71 patients, with a cumulative incidence of 55.2% (95% CI, 42.7 to 66.0) at 1 year. New-onset hypogammaglobulinemia occurred in 18/64 patients, with a cumulative incidence of 28.4% (95% CI, 17.8 to 39.8) at 1 year. The cumulative incidence of hypogammaglobulinemia was significantly higher in the proven PTLD cohort (53.8% [95% CI, 22.3 to 77.5]) than in the probable PTLD (24.5% [95% CI, 9.6 to 42.9]) and viremia (19.4% [95% CI, 6.9 to 36.8]) cohorts (p=0.03).
This study shows that rituximab is effective and clears EBV viremia in >95% of CD34+ selected SCT recipients with EBV viremia or probable PTLD. However, only 22.2% of patients with proven PTLD achieved a CR. Hypogammaglobulinemia and neutropenia are important toxicities of rituximab. Infectious complications were common, but difficult to attribute solely to rituximab. Further research and prolonged follow-up are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal