Abstract
Background: Although curative treatment for acute myeloid leukemia, the success of allogeneic hematopoietic cell transplantation (HCT) is limited due to leukemia relapse and graft-versus-host disease (GvHD). Rabbit anti-thymocyte globulin (ATG) used for GvHD prophylaxis does not increase relapse (Walker et al: Lancet Oncol 2016). The non-increase in relapse could be because ATG has a direct anti-leukemic effect (Dabas et al: BBMT 2016). Multiple studies have suggested that a high number of leukemic stem cells (LSCs, also called leukemia initiating cells) remaining after therapy is associated with relapse. Therefore, targeting LSCs may be important for treating or preventing relapse. We demonstrated ATG's cytotoxic effect against acute myeloid leukemia (AML) blasts (Dabas et al: BBMT 2016). However, ATG's effect on LSCs has not been evaluated. In this study, we investigated in vitro ATG-induced complement-independent cytotoxicity (CIC, presumably direct induction of apoptosis) and complement-dependent cytotoxicity (CDC) against LSCs. This was also compared to ATG-induced CIC and CDC against healthy hematopoietic stem cells (HSCs).
Methods:Frozen peripheral blood mononuclear cells (PBMNCs) from 15 patients newly diagnosed with AML were used as the source of LSCs. Frozen mononuclear cell apheresis samples from 15 healthy stem cell donors were used as the source of HSCs. We measured by flow cytometry CIC and CDC induced by ATG at 10 mg/L (the median peak concentration achieved with 4.5 mg/kg ATG given over day -2, -1 and 0) and 50 mg/L. CIC was induced by incubating cells with ATG in the presence of heat-inactivated (complement-depleted) fetal bovine serum for 4 hours. As a negative control for the calculation of background, cells were cultured under the same conditions except without ATG. CDC was induced by incubating cells with ATG in the presence of human serum as a source of complement for 15 minutes. As a negative control (for background), cells were incubated with ATG in the presence of heat-inactivated human serum (no complement). The LSCs and HSCs were phenotypically defined as CD45dim/neg, side scatterlow, CD34+, CD38neg and negative for CD14, CD16, CD19, CD56, CD235a and CD41a. For CIC, dying/dead cells were identified as Annexin V positive (Annexin V+). For CDC, dead cells were identified as 7-amino-actinomycin D positive (7AAD+).
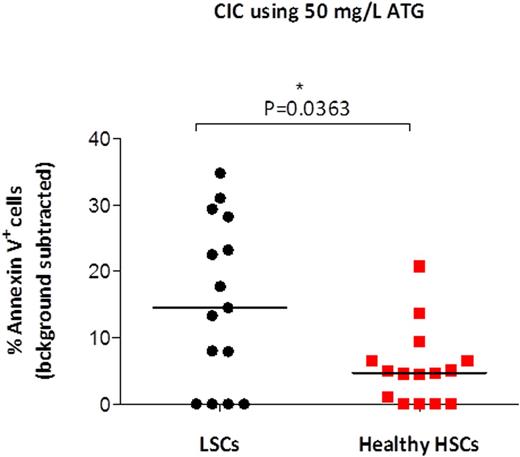
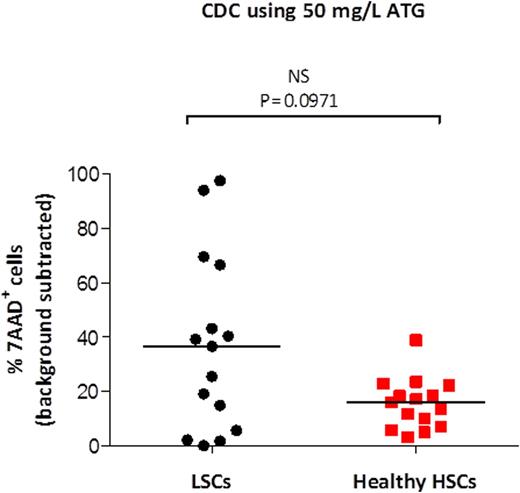
Results: ATG induced both CIC and CDC of LSCs at the concentration of 50 mg/L but not 10 mg/L. For CIC, the median percent Annexin V+ LSCs after incubation with 50 mg/L ATG was 22.3% vs 18.4% for background (p=0.0043, Wilcoxon matched pairs test). For CDC, the median percent 7AAD+ LSCs after incubation with 50 mg/L ATG was 37.2% vs 2% background (p=0.0004, Wilcoxon matched pairs test). Next, we compared the cytotoxicity of 50 mg/L ATG against LSCs versus healthy HSCs. For CIC, a significantly greater percent of LSCs than HSCs was killed (P=0.0363, Mann-Whitney rank sum test) (Figure 1). Similarly, for CDC, there was a trend toward a greater percent of LSCs than HSCs killed (P=0.0971) (Figure 2).
Conclusion: ATG kills LSCs in vitro via both CIC and CDC. However, the degree of the killing is minor and only observed at a higher ATG concentration than typically achieved in patients. Our data also suggest that if high dose ATG was used in patients (resulting in ≥50 mg/L concentration), LSCs could be killed to a greater degree than healthy HSCs.
LSCs are more sensitive to ATG mediated CIC than healthy HSCs. Mononuclear cells from 15 patients newly diagnosed with AML and cells from leukapheresis products of 15 healthy stem cell donors were incubated with ATG (50 mg/L) in the absence of complement. After 4 hours, the percentage of dead or dying (Annexin V+) cells was measured in LSCs and HSCs. Adjusted percentage (background subtracted) is shown for each patient/donor. Medians are shown as horizontal lines.
LSCs are more sensitive to ATG mediated CIC than healthy HSCs. Mononuclear cells from 15 patients newly diagnosed with AML and cells from leukapheresis products of 15 healthy stem cell donors were incubated with ATG (50 mg/L) in the absence of complement. After 4 hours, the percentage of dead or dying (Annexin V+) cells was measured in LSCs and HSCs. Adjusted percentage (background subtracted) is shown for each patient/donor. Medians are shown as horizontal lines.
LSCs are more sensitive to ATG mediated CDC than healthy HSCs. Mononuclear cells from 15 patients newly diagnosed with AML and cells from leukapheresis products of 15 healthy stem cell donors were incubated with ATG (50 mg/L) in the presence of complement. After 15 minutes, the percentage of dead (7AAD+) cells was measured in LSCs and HSCs. Adjusted percentage (background subtracted) is shown for each patient/donor. Medians are shown as horizontal lines.
LSCs are more sensitive to ATG mediated CDC than healthy HSCs. Mononuclear cells from 15 patients newly diagnosed with AML and cells from leukapheresis products of 15 healthy stem cell donors were incubated with ATG (50 mg/L) in the presence of complement. After 15 minutes, the percentage of dead (7AAD+) cells was measured in LSCs and HSCs. Adjusted percentage (background subtracted) is shown for each patient/donor. Medians are shown as horizontal lines.
Daly:Sanofi-Genzyme: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal