Abstract
A growing body of data supports the benefit of first-line rituximab therapy for EBV- associated post-transplant lymphoproliferative disorder (PTLD) the potential for adoptive cellular therapy, and the benefit of early diagnosis. Quantitative PCR has proven to be an important, albeit not infallible, diagnostic test; however, data regarding the utility We reviewed the records of 731 adult patients who underwent allogeneic hematopoietic cell transplantation (HCT) at Stanford University Medical Center from January 2010 through Feb 2016.
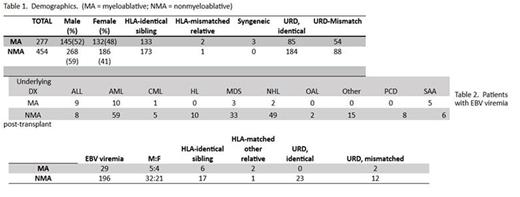
(Table 1. Demographics) With respect to EBV screening, all patients received 400 mg po acyclovir tid for one year, preemptive strategies based on plasma PCR for CMV were used with either IV ganciclovir (MA) or valganciclovir (NMA) administered. Quantitative EBV PCR was performed on whole blood (Jan 2010 through mid-2011) or plasma (mid-2011 to present) every other week in asymptomatic patients or at any time a patient developed symptoms that may be attributed to EBV.
Observations/Conclusions:
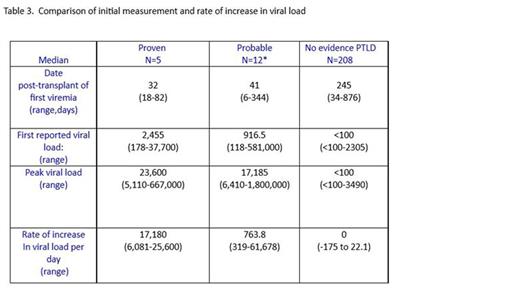
· Both the initial measureable and the rate of increase in viral load were significantly greater in patients with PTLD than those with isolated viremia.
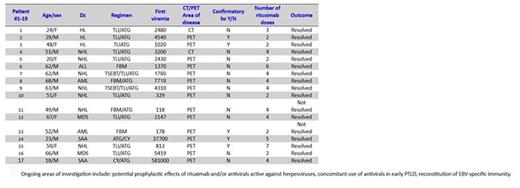
· Promptly administered rituximab was effective in improving resolution of PTLD when compared to historically reported data.
· Conversely, patients with low grade viremia without increase in viral load may be watched expectantly.
· Although not standard practice, importance of EBV serology of donor has been demonstrated in SOT and pediatric HCT and would be anticipated to be of equal importance in adults, especially
· Early biopsy or flow cytometric analysis of EBV+ lymphocyte to determine polymorphic versus monomorphic disease can further refine our understanding the pathophysiology of disease
· The crucial limitation of our study is that determination of EBV serology was not routine; however, by limiting our analysis to patients with EBV reactivation and the use of a standardized protocol to respond to viremia has permitted the current observations to be made.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal