Abstract
Background: Patients undergoing allogeneic hematopoietic stem cell transplant with reduced-intensity conditioning (RIC-alloSCT) rely primarily on the graft-versus-tumor (GVT) effect to prevent relapse. Although relapse remains high in this setting, previous data suggest modifications of allograft composition could produce enhanced GVT effect and improve outcomes. Prior retrospective studies suggested higher total nucleated cell (TNC) and CD8 dose correlate with improved overall survival (OS) and a reduction in relapse among all patients undergoing RIC-alloSCT. The aim of our study was to further investigate this association by comparing transplant outcomes with detailed graft immunophenotyping data including T-cell and NK-cell activation and maturation status and CD34 cell doses in patients with AML and MDS undergoing RIC-alloSCT.
Methods: We performed a retrospective analysis using data from consecutive patients with AML and MDS who underwent RIC-alloSCT at a single center from 2010-2015 who had graft immunophenotyping data available. We compared transplant outcomes with TNC per kilogram (kg) and dose of CD3, CD4, CD8, CD34, and NK cells along with selected activation and maturation subsets. A competing risk regression analysis was conducted to examine the association between cell dose/kg and risk of relapse and graft versus host disease (GVHD). Overall survival and relapse free survival (RFS) were assessed utilizing the Cox proportional hazard model. Estimates of survival probability were determined by the Kaplan-Meier survival function. The log-rank test was used to compare OS and RFS among patients. Univariable and multivariable regression analyses were performed. Disease Risk Index (DRI) was used to stratify risk among patients. To identify optimal cutoffs (OC) of continuous variables for a specific outcome, a Classification and Regression Tree (CART) algorithm was utilized.
Results: Our study included 142 patients who underwent RIC-alloHSCT for AML (n=97, 68%) and MDS (n=45, 32%). The vast majority of patients (98%) received fludarabine and busulfan conditioning with 65% receiving anti-thymocyte globulin. All patients received tacrolimus-based GVHD prophylaxis. Overall relapse rate was 37% at 3 years. Overall survival was 49% with median follow-up of 3.2 years.
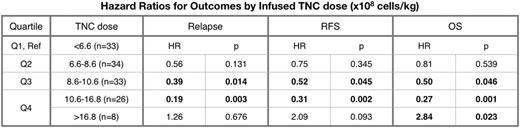
Total Nucleated Cell Dose: In multivariable analysis controlling for disease, comorbidity index, and performance status, a high infused TNC dose (10.6 - 16.8 x 108 cells/kg) correlated with improved overall survival (HR 0.27, 95% CI [0.12 - 0.59], p=0.001) and relapse free survival (HR 0.31, 95% CI [0.15 - 0.64], p=0.002). However, a very high TNC dose (OC >16.8 x 108 cells/kg) correlated with significantly worse OS (HR 2.84, 95% CI [1.16 - 6.98], p=0.023) and trended toward worse RFS (HR 2.09, 95% CI [0.88 - 4.93], p=0.093). Higher TNC doses were also correlated with an increased risk of chronic GVHD (HR 2.16, 95% CI [1.28 - 3.67], p=0.004, OC 9.8 x 108 cells/kg).
Cell Subsets: Very low CD3 cell doses (OC <1.06 x 108 cells/kg) correlated with worse OS (HR 3.30, 95% CI [1.23 - 8.85], p=0.017) and worse RFS (HR 2.64, 95% CI [1.01 - 6.88], p=0.047). High CD3 doses were correlated with an increase in acute GVHD (all grades) (HR 2.41, 95% CI [1.36 - 4.25], p=0.002, OC 4.1 x 108 cells/kg). NK, CD4, CD8, and CD34 cell dose was not associated with relapse, OS, or RFS in multivariable analysis. Utilizing a previously published (Reshef et al, JCO, 2015) cutoff of 7.2 x 108 cells/kg, there was a trend toward a reduction in relapse with higher CD8 cell dose, but this did not reach statistical significance (HR 0.67, 95% CI [0.39 - 1.16], p=0.15). A high dose of late activated T-cells (CD3+/HLA-DR+) correlated with increased risk of acute GVHD (HR 2.31, 95% CI [1.26 - 4.20], p=0.006, OC 2.01 x 107 cells/kg) and chronic GVHD (HR 1.85, 95% CI [1.12 - 3.07], p=0.016, OC 6.6 x 106 cells/kg).
Conclusion: Among AML and MDS patients following RIC-alloSCT, higher infused TNC/kg was associated with improved RFS and overall survival, confirming findings from a recent study (Martin et al, Haematologica, 2016). However, very high TNC doses may be deleterious. T-cell activation state influences risk of GVHD. Further research needs to be pursued to corroborate these findings and potentially optimize TNC and T-cell doses. We were unable to identify a significant correlation between CD8 cell dose and relapse or overall survival.
Lozanski:Genentech: Research Funding; Boehringer Ingelheim: Research Funding; Stemline Therapeutics Inc.: Research Funding; Beckman Coulter: Research Funding. Andritsos:Hairy Cell Leukemia Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal