Abstract
Introduction: Despite the successes of treatment with tyrosine kinase inhibitors (TKIs) in patients with CML, allogeneic hematopoietic stem cell transplantation (ASCT) continues to be a potentially curative option for patients with advanced disease of who fail TKI therapy. Here we analyzed outcomes of patients with advanced CML (aCML) (beyond first chronic phase-CP1) who received an ASCT at our institution to identify factors associated with improved survival.
Methods:207 consecutive patients withaCML treated at The University of Texas MD Anderson Cancer Center after year 2000 were included. The median age was 44 years (range 2-70 years), 135 (65%) were male, 77% had less than 5% bone marrow (BM) blasts, 129 (65%) had persistentPh-chromosome positive, and 176 patients (85%) were less than a major molecular response at transplant. Forty of 114 tested patients (35%) had resistant BCR-ABL mutations and 10 patients (8.7%) had T315I mutation at transplant. Conditioning regimen wasmyeloablative (MAC) in 140 patients (68%). Donors were matched related (MRD), matched unrelated (MUD),haploidentical (HAPLO), mismatched unrelated (MMUD), umbilical cord blood (UCB) and 1Ag mismatched related (MMRD) in 79 (38%), 75 (36%), 18 (9%), 17 (8%), 11 (5%) and 7 (3%) patients, respectively. The median time from diagnosis to transplant was 27 months (range 1-318 months) while the median follow-up duration was 20 months (range 1-194 months).
Results:At 30 days post-transplant, 180 of 200 tested patients (90%) and 134 of 201 tested patients (67%) achieved a complete cytogenetic and at least a major molecular response, respectively. The response to transplant by day 30assessment correlated significantly with the disease status before transplant. A higher percentage of patients who experienced cytogenetic response before transplant experienced molecular response post-transplant (77%) compared with those who did not (61%; p=0.027). For the entire group, the 1-year cumulative incidence (CI) of acute GVHD (aGVHD) grade II-IV and grade III-IV were 41% and 15%, respectively; 5-year CI of extensive chronic GVHD (cGVHD) was 31%.Haploidentical transplant patients had lesscGVHD compared with HLA matched donor transplants (14% vs. 32% for HLA matched transplants). The CI of non-relapse mortality at 100 days and 1 year was 14% and 30%, respectively. Sixty-five patients (31%) had molecular relapse after transplant, which correlated with the degree of disease control before transplant. The CI of cytogenetic and molecular relapse at 5 years was 22% and 31%, respectively.
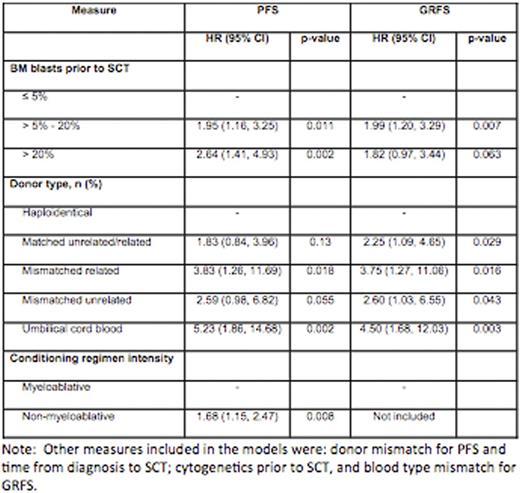
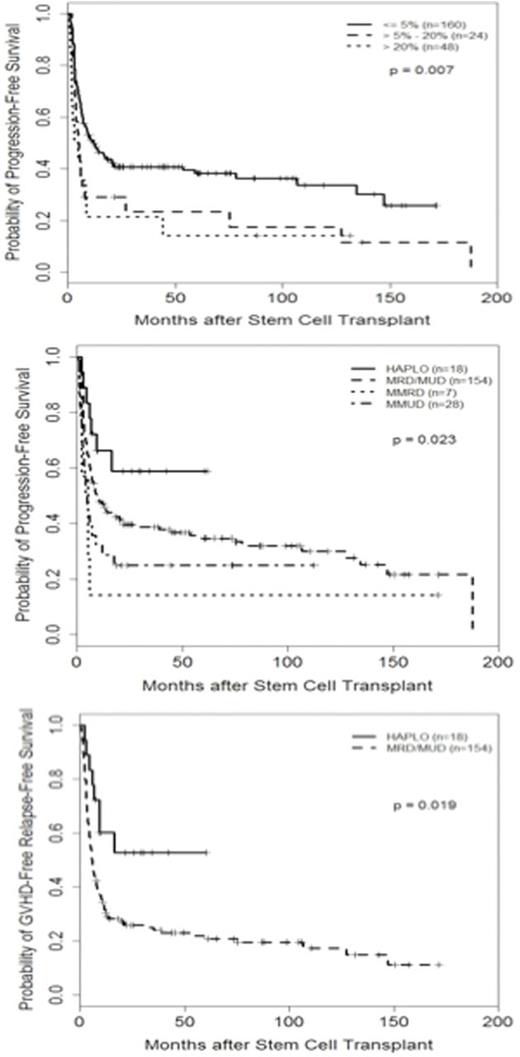
Overall the 5-year survival (OS), progression free survival (PFS) and GVHD-free, relapse-free survival (GRFS)was49%, 34%, and 22%, respectively. Adjusting for all significant measures, percentage of BM blasts before transplant and donor type were significantly associated with PFS and GRFS (Table1, Figure 1).Haplodenticaltransplant patients had longer PFS and GRFS compared with other donor types including matched related or unrelated donor (5-year GRFS for HAPLO, MRD, MUD, MMUD, UCB and MMRD were 53%, 19%, 23%, 29%, 9%, and 0%, respectively; p=0.033) (Table 1, Figure 1). Cytogenetic and molecular response before transplant as well as year of transplant did not predict survival after transplant.
Conclusions: ASCT is curative for a proportion of patients withaCML. PFS and GRFS are favorably influenced by percentage of BM blasts and donor type, withhaploidentical donor having at least as good outcomes as HLA matched donors, while molecular and cytogenetic response before transplant do not appear to correlate with survival post-transplant.
PFS and GRFS based on percentage of BM blasts before transplant and donor types
Cortes:Arog: Research Funding; Teva: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Astellas: Research Funding; Ambit: Research Funding. Champlin:Intrexon: Equity Ownership, Patents & Royalties; Ziopharm Oncology: Equity Ownership, Patents & Royalties. Ciurea:Spectrum Pharmaceuticals: Other: Advisory Board; Cyto-Sen Therapeutics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal