Abstract
Introduction
Chronic graft-versus-host disease (cGVHD) remains a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Perturbations in immune reconstitution following allo-HSCT are believed to be integral to the pathophysiology of cGVHD. We hypothesized that skewing in T cell repertoire around day 80 would be predictive of cGVHD, while maintenance of a broad repertoire would be protective. One method used to assess skewing is the Gini Index, a conventional measure of income inequality. In this prospective study, we utilized a rapid FACS based method of TCR Vβ repertoire analysis within CD4+ and CD8+ in patients at day 80-110 post allo-HSCT to determine whether preferential TCR skewing predicts the development of cGVHD.
Methods
Peripheral blood samples were obtained from 79 consented patients at day 80-110 following allo-HSCT at The University of Texas MD Anderson Cancer Center from October 2012 to November 2015. All patients were in remission of their disease at the time of blood sampling. Fresh PBMC were analyzed by FACS after staining for CD3, CD4, CD8 and IOTest Beta Mark 24 TCR Vβ kit. The TCR Gini Index scores (GS) were calculated for the T cell subsets, and ranged from 0 to 100. Low GS indicate more equal distribution of TCR-Vβ families (i.e. broad repertoire), whereas high GS reflect unequal distribution of TCR-Vβ families (i.e. repertoire skewing). Predictive thresholds of GS were identified in quartiles analysis and confirmed by Receiver Operating Characteristic (ROC) curve.The association between GS and the rate of cGVHD since the date of blood sampling was evaluated using competing-risks regression analysis. The diagnosis of cGVHD was based on the 2014 National Institutes of Health guidelines.
Results
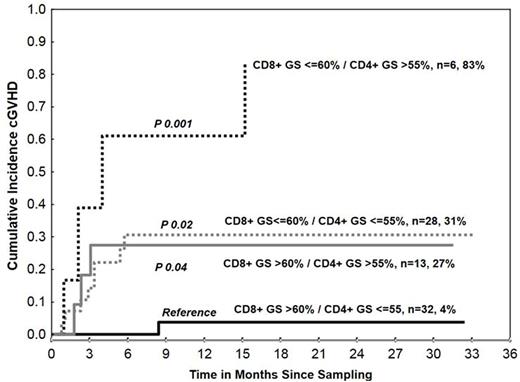
The median age at transplant was 53 years (range 21-75) and 60% of patients were male. The most common transplant indication was AML/MDS (52%) followed by lymphoma (16%), ALL (14%), and other (18%). Graft source was peripheral blood (54%), bone marrow (43%) and umbilical cord blood (UCB) (3%) with donors being matched unrelated (65%), matched-related (24%), mismatch related (9%) and UCB (3%). Forty three (54%) patients were not in remission at the time of transplant. At the time of enrollment, 47% had history of grade II-IV acute GVHD and 44% were on systemic steroids. At a median follow up of 2 years since blood sampling, 16 patients developed cGVHD (cumulative incidence (CI) 23%; 95% Confidence Interval: 15-35). The median time from HSCT to cGVHD was 174 days (range 111-551 days). None of the clinical or demographic characteristics listed above, except history of acute GVHD, was significantly associated with the incidence of cGVHD. GS were significantly predictive of the incidence of cGVHD. The 2 year %CI of cGVHD was 47% in patients who had CD4+ TCR GS >55% (n=19) compared with 16% in those with CD4+ GS ≤55% (n=60) (hazard ratio (HR) =3.4, P=0.01). In contrast to CD4+, lower CD8+ GS predicted for higher incidence of cGVHD. The 2 year %CI of cGVHD was 39% in patients (n=34) with CD8+ GS ≤60% compared with 10% in patients (n=45) with CD8+ GS >60% (HR=4.5, P=0.009). When both CD4+ and CD8+ GS were considered in combination, the 2 year incidence of cGVHD was highest in patients (n=6) with both low CD8+ (≤60%) and high CD4+ (>55%) GS, and lowest in patients (n=32) with high CD8+ (>60%) and low CD4+ (≤55%) GS (%CI: 83% vs 4%, P=0.001). The combined effect of high CD4+ and low CD8+ TCR skewing was more pronounced in patients with a history of grade II-IV acute GVHD. Low CD8+ (≤60%, n=28) or high CD4+ (>55%, n=13) GS occurring separately were associated with comparable rates of cGVHD (%CI: 31% and 27%, p=0.8), irrespective of history of grade II-IV acute GVHD (Figure).
Conclusions
High CD4+ and low CD8+ TCR Gini indices were independent predictors of cGVHD. Our results indicated that the higher skewing of CD8+ TCR appeared to be protective of cGVHD and the opposite for CD4+. Our study is the first to test the usefulness of FACS-based TCR measurement and Gini Index score in a clinical setting to help predict the development of cGVHD and provide greater insights into the pathogenesis of cGVHD. Using this convenient FACS-based method to identify patients who are at risk of developing cGVHD could be beneficial from development of effective preventive or therapeutic strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal