Abstract
INTRODUCTION
The use of high-dose cyclophosphamide as post-transplant Graft versus Host Disease (GvHD) prophylaxis has revolutionized haploidentical hematopoietic stem cell transplantation (haplo-HSCT), allowing the safe infusion of T cell replete grafts. The efficacy of post-transplant cyclophosphamide (PT-Cy) has its basis in its capacity to selectively eliminate proliferating cells, including alloreactive T cells. It is however to date unknown whether PT-Cy affects the reconstitution of Natural Killer (NK) cells, whose alloreactivity is known to play a major role in T cell-depleted haplo-HSCT.
PATIENTS AND METHODS
We analyzed the grafts and serial peripheral blood (PB) and bone marrow (BM) samples from 14 patients who received T cell replete haplo-HSCT followed by PT-Cy at the San Raffaele Scientific Institute, Milan (n=10, OSR) or the Johns Hopkins University, Baltimore (n=4, JHU). OSR patient received a myeloablative conditioning, PB stem cell grafts, and sirolimus and mycophenolate as pharmacological GvHD prophylaxis. JHU patients received the "classical" Baltimore nonmyeloablative conditioning, unmanipulated BM grafts, and tacrolimus and mycophenolate as pharmacological GvHD prophylaxis.
To characterize NK cells reconstitution, we monitored absolute counts and employed a 27-marker flow cytometry panel with high dimensional single-cell analysis using the bh-SNE algorithm.
We used intracellular staining to determine the frequency of Ki67+ proliferating cells and expression of Aldehyde Dehydrogenase (ALDH), known to confer resistance to PT-Cy. Interleukin-15 (IL-15) serum concentration was quantified using the Bio-Plex Pro Human Cytokine 4-plex assay.
To directly assess the effect of PT-Cy on proliferating NK cells we exposed graft NK cells to IL-15 and mafosfamide, a cyclophosphamide analogue active in vitro. Functional assays against leukemic cell lines and primary AML blasts were performed measuring CD107A degranulation on NK cells and Annexin V on targets.
RESULTS
All patients received high numbers of mature donor NK cells as part of the graft (OSR median 17x106/kg, JHU median 7.25x106/kg ), and donor-derived NK cells were detectable as early as day 3 after HSCT and throughout the entire follow-up.
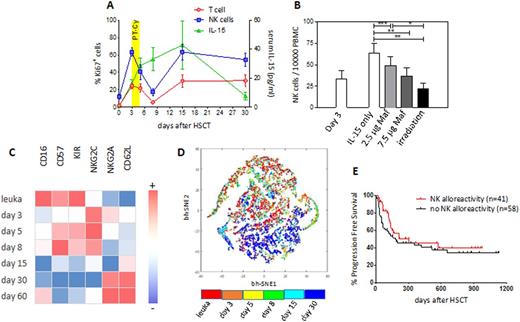
At day 3 after HSCT, all subsets of NK cells, including single KIR+ alloreactive cells, were actively proliferating (mean 61.23% of Ki-67+ cells for OSR patients, and 58% for JHU patients), possibly driven by the high levels of IL-15 detected in patient serum after conditioning (Fig.1A).
After PT-Cy, a marked reduction in the frequency and counts of proliferating NK cells was evident irrespectively of the transplantation platform, suggesting selective killing of dividing cells by PT-Cy.
In line with this hypothesis, NK cells from the graft and from patient PB at day 3 after HSCT showed no detectable ALDH expression, and NK cells prompted to proliferate in vitro were killed in a dose-dependent manner by mafosfamide (Fig.1B).
The phenotype of NK cells also changed upon PT-Cy: whereas before the infusion they resembled their mature counterparts from the graft, after PT-Cy an immature phenotype, CD62L+NKG2A+KIR-, became prevalent, suggesting derivation from donor HSCs rather than from infused NK cells (Fig.1C). Accordingly, bhSNE maps demonstrated differential clustering of NK cells from the graft and analyzed 30 days after HSCT (Fig.1D).
In line with these features, we detected very low numbers of putatively alloreactive single KIR+ NK cells both in the PB and in the BM of patients at day 30 after HSCT, and these NK cells displayed impaired cytotoxic potential against leukemic targets.
Finally, consistent with these observations, when we analyzed the impact of predicted NK alloreactivity in an extended series of 99 patients who received myeloablative haplo-HSCT with PT-Cy, we detected no significant difference in progression-free survival (Fig.1E).
CONCLUSION
Our data suggest that the majority of mature NK cells infused with unmanipulated grafts are eliminated upon PT-Cy administration and that in this transplantation platform NK cell alloreactivity might be blunted by the elimination of donor single KIR+ NK cells and by the competition between reconstituting NK and T cells. Still, the high levels of IL-15 detected in patients' sera at early time-points might provide a biological rationale for the infusion of mature donor NK cells early after PT-Cy administration.
Bonini:TxCell: Membership on an entity's Board of Directors or advisory committees; Molmed SpA: Consultancy. Ciceri:MolMed SpA: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal