Abstract
Adoptive cell therapy employing T cells equipped with a chimeric antigen receptor (CAR) containing a single chain antibody fragment fused to T cell signaling domains 4-1BB and CD3zeta (CTL019) has shown great potency against various hematopoietic malignancies, e.g. B cell acute lymphoblastic leukemia (ALL). However, it has not shown the same response rate in other malignancies such as chronic lymphocytic leukemia (CLL). We recently demonstrated that the in vivo expansion and persistence of CAR T cells is an important predictor of response to CTL019 in CLL (PMID: 26333935) and ALL (Thudium et al., ASH 2016; Fraietta et al., ASH 2016). Furthermore, it is well known that prolonged culture of T cells negatively impacts the in vivo expansion of the adoptively transferred cells. We therefore hypothesized that minimizing the ex vivo manipulation of T cells would improve the efficacy of CAR T cells.
We tested this hypothesis by generating CART19 cells using our standard 9-day manufacturing process plus two abbreviated versions. Cells from normal donors (n=9) and from patients with adult ALL (n=6) were stimulated on day 0 followed by transduction with the CAR19-encoding lentiviral vector on day 1. Cells were harvested on days 3, 5, and 9. Cryopreserved aliquots were evaluated for T cell differentiation using polychromatic flow cytometry, cytokine secretion profile using Luminex, cytolytic ability against a leukemia cell line (NALM6), proliferative ability upon restimulation with CD19-expressing target cells, and in vivo control of our well-established xenogeneic ALL model employing NALM6 as the target. Our data show that all cultures contain a substantial proportion (40%-80%) of na•ve-like CD45RO-CCR7+ T cells that progressively differentiate leading to the accumulation of predominantly (60%-90%) central memory T cells by the end of expansion. Comparative assessment of the CART19 cells at all three time points demonstrated that the cells from the shorter cultures displayed a superior in vitrocytolytic activity, and proliferative response compared to the standard process. In addition,the cells from our standard and shortened cultures all secreted comparable levels of type I cytokines (i.e. IFN-g, IL-2, and TNF-α).
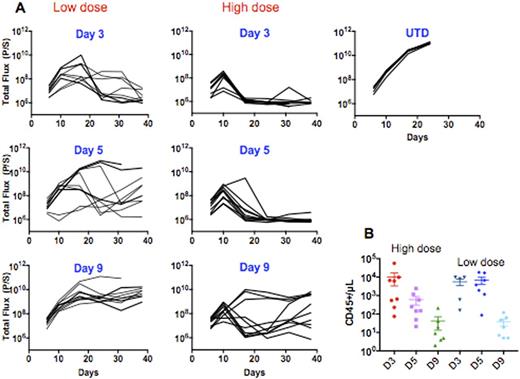
Importantly, we investigated the therapeutic potential of cells harvested at day 3 versus later time points. We treated NALM6 xenograftmice with a low dose (0.5 x106 CAR+ T cell I.V.) or standard dose (3 x106 CAR+ T cell I.V.).We demonstrate that day 3 CART19 cells show superior anti-leukemic activity compared to day 5 or day 9 cells. Additionally, we show that mice treated at a low dose with day 3 cells exhibit the greatest anti-leukemic efficacy compared with day 9 cells where the latter fail to control leukemia (Figure 1).
Our preclinical findings provide evidence that extended ex vivo manipulation of T cells negatively affects their in vivo potency.In summary, we show that limiting T cell culture ex vivo to the minimum required for lentiviral transduction provides the most efficacious T cells for adoptive T cell immunotherapy.
Ghassemi:Novartis: Research Funding. Scholler:Novartis: Patents & Royalties; University of Pennsylvania: Patents & Royalties: FAP-CAR US Patent 9,365,641 for targeting tumor microenvironment. Nunez-Cruz:Novartis: Research Funding. Barrett:Novartis: Research Funding. Bedoya:Novartis: Patents & Royalties. Fraietta:Novartis: Patents & Royalties: Novartis, Research Funding. Lacey:Novartis: Research Funding. Levine:GE Healthcare Bio-Sciences: Consultancy; Novartis: Patents & Royalties, Research Funding. Grupp:Novartis: Research Funding. June:Johnson & Johnson: Research Funding; Tmunity: Equity Ownership, Other: Founder, stockholder ; University of Pennsylvania: Patents & Royalties; Pfizer: Honoraria; Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; Immune Design: Consultancy, Equity Ownership; Celldex: Consultancy, Equity Ownership. Milone:Novartis: Patents & Royalties, Research Funding. Melenhorst:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal