Abstract
Angiogenesis and inflammation are two closely related processes and inhibition of angiogenesis can ameliorate inflammatory diseases by reducing the recruitment of tissue infiltrating leukocytes. However, there is limited evidence on initial mechanisms of both processes and it is unknown if angiogenesis contributes to initiation of inflammation or is a mere consequence of inflammation.
Recent research showed that graft-versus-host disease (GVHD) is associated to angiogenesis making the endothelium a potential new therapeutic target during allogeneic stem cell transplantation (bone marrow transplantation, allo-BMT).
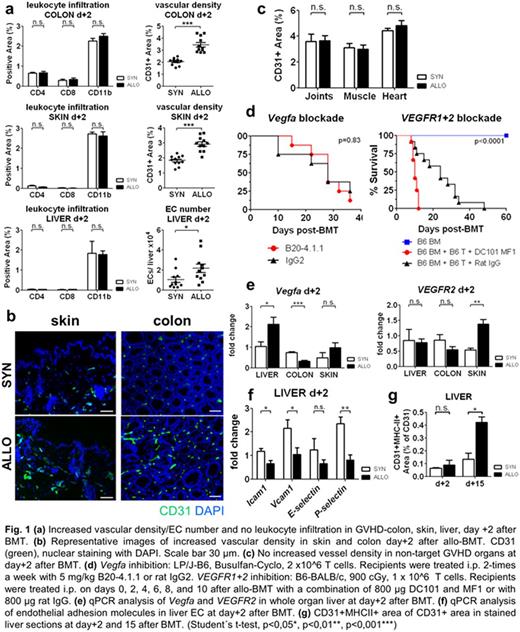
We studied kinetics and quantity of angiogenesis and leukocyte infiltration in murine acute GVHD models (LP/J→B6, B6→Balb/c, B6→BDF and 129S2/Sv→B6) by assessing CD31, CD4, CD8, CD11b by immune staining and FACS analysis. In GVHD target organs colon, skin and liver, we found significant increased vascular density and endothelial cell (EC) number but no leukocyte infiltration as early as day+2 after BMT (Fig. 1a,b). Leukocyte infiltration occurred secondary to angiogenesis at day+7 or later. No angiogenesis and inflammation occurred in the non-acute GVHD target organs joints, cardiac and skeletal muscle (Fig. 1c) suggesting a previously unrecognized role of the endothelium in GVHD organ tropism. Similar analyses in a standard model for inflammatory bowel disease, the 3% dextran sulfate sodium-induced (DSS) colitis, confirmed that angiogenesis preceded leukocyte infiltration in colon (d+1 vs. d+3) implicating that the significance of our results surpasses the field of transplantation biology.
We aimed at identifying pathways that are relevant for initiation of angiogenesis during GVHD and first investigated the Vegfa/VEGFR1+2 axis. Based on our previous observation demonstrating a reduction of GVHD due to inhibition of neovascularization by anti-VE-cadherin antibodies (Penack et al, JNCI 2010), we performed therapeutic interventions with monoclonal blocking antibodies. Anti-VEGFR1+2 (DC101+MFI, Imclone) and anti-Vegfa (B20-4.1.1, Genentech) did not lead to GVHD reduction (Fig. 1d). Furthermore, we found no consistent upregulation of Vegfa and VEGFR2 expression levels in GVHD target organs during initiation of GVHD or later time points (Fig. 1e) suggesting that the Vefga/VEGFR2 pathway is not a major mechanism for initiation of angiogenesis in GVHD target organs.
We next assessed known endothelial activation signs and performed qPCR analysis of adhesion molecules Icam1, Vcam1, E- and P-selectin (Fif. 1f) as well as immune staining of MHC-II (Fig. 1g). We confirmed previous knowledge that endothelial cells upregulate adhesion molecules and MHC-II during established GVHD. In sharp contrast, adhesion molecules and MHC-II were downregulated or not changed during the initiation of angiogenesis at day+2. This suggests that alternative pathways are important during initial angiogenesis in early GVHD.
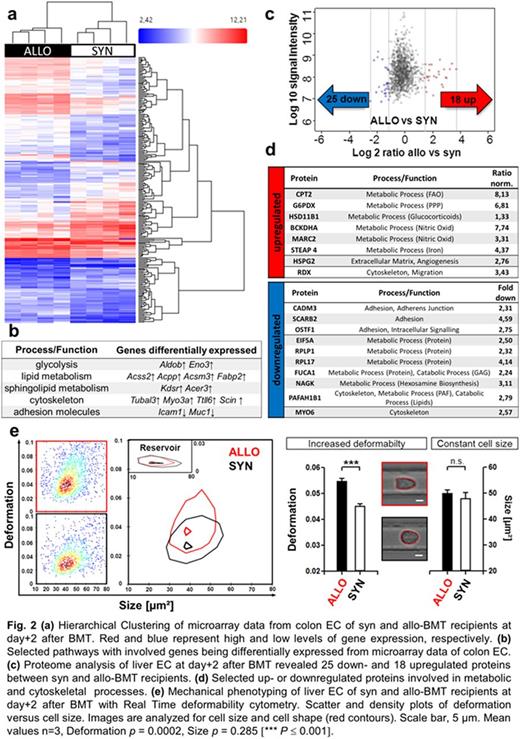
To identify alternative pathways during initial angiogenesis in an unbiased approach, we performed microarray analyses of FACS-sorted colon ECs (Fig. 2a) and LC-MS/MS proteome analyses of MACS-isolated liver ECs (Fig. 2c) at day+2 after allo-BMT vs. syn-BMT. Strikingly, we detected substantial metabolic and cytoskeletal changes in ECs of allo-BMT recipients (Fig. 2b,d). Recently, metabolic processes have been shown to be associated with cytoskeletal remodeling during EC migration and angiogenesis (Uebelhoer et al., J Vasc Res 2016). Accordingly, measuring single-cell mechanical characteristics with Real-Time deformabiliy cytometry (Otto et al., Nature Methods 2015) revealed profoundly higher deformation (D = 0.0547 ± 0.0011) and overall softening of ECs from allo-BMT recipients (Fig. 2e) indicating to enhanced EC migration and proliferation.
We demonstrate that angiogenesis initiates GVHD in target organs and is restricted to target organs. Our findings implicate that endothelial biology plays a major role in GVHD organ tropism and disease development. We revealed novel genes and proteins regulating migration and proliferation of EC in initial angiogenesis during GVHD. Our study amends the knowledge on the interplay between the vasculature and inflammation opening a new window to develop therapeutic strategies targeting the endothelium.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal