Abstract
Background: According to VISTA study, the prognosis of elderly multiple myeloma (MM) patients has been improved with VMP therapy. However, intensive chemotherapy has induced severe adverse events (AEs), resulting in highly discontinued rate, especially often seen in unfit or frail patients. Several studies have demonstrated the potent of weekly bortezomib administration in induction therapy (GIMEMA, GEM2005MAS65), and maintenance therapy with bortezomib. Based on these fact, we designed phase 2 clinical study, BoRtezomib-based Optimized therapy Aiming Disease control in Japan (BROAD-J study), based on a weekly VMP as induction therapy followed by maintenance therapy with bortezomib (Bor-MT) with every two weeks administration for newly-diagnosed (ND) symptomatic non-transplant eligible MM patients. (UMIN 00007335)
Method: From August 2011 to June 2016, according to the International Myeloma Working Group (IMWG) criteria, 87 patients older than 65 years with symptomatic MM were eligible for this trial (Table 1). The primary objectives were included maximum response and time to progression duration. Secondary objective was the continued treatment duration and adverse event rate. The induction phase consisted of VMP treatment: Melpharan: on days 1-4 (6 mg/m2), every 35-day cycle; prednisolone: on days 1-4 (40mg/d, every 35-day cycle; Bortezomib: 1.3mg/m2, d1, 8, 15, 22 every 35-day cycle. After 9 cycles of VMP therapy, the maintenance therapy starts. The maintenance phase was consisted of twice a month bortezomib administration (1.3 mg/m2) without dexamethasone until progressive disease. Dose reductions of bortezomib were allowed according to instructorfs recommendation. Patients could receive supportive therapy including bisphosphonates and transfusions as necessary. AEs were graded according to NCI-CTCAE v4.0. Response was assessed prior to every treatment cycle. Response categories were based on the International Myeloma Working Group uniform response criteria.
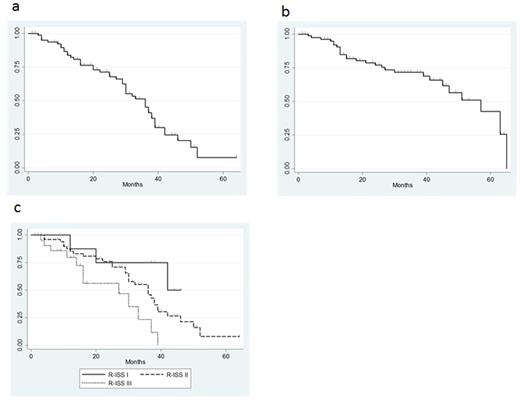
Results: Mean age was 75 years (66-88), sex ratio was 44:43 (M: F), and ISS stage was I 12%, II 43%, III 43%. High risk cytogenetics abnormalities (i.e. del17p, t(14;16)) were observed in 13 patients (15%). Overall response rate was 87% and complete response was obtained in 22 patients (25%) (Table 1). Median progression free survival (PFS) was 36 months (Figure 1a) and median overall survival was 57 months (Figure 1b). Median PFS (Kaplan-Meier estimate) was significantly higher with patients who obtained CR or VGPR than PR or SD (P<0.0001) (Figure 1c). Significantly higher ORR, rate of VGPR or better, and rate of CR or better were achieved in patients with revised ISS I stage, respectively. In induction phase, 32% of patients were suffered from greater than grade 3 AEs, whereas only 3% had grade 3 or 4 AEs in maintenance phase.
Discussion: This trial reveals that VMP regimen with weekly bortezomib administration is effective and tolerablefor unfit or frail elderly MM patients. In addition, maintenance therapy of twice a month bortezomib administration was effective in patients who obtained VGPR or more better. These results are no way inferior to results of VISTA trial. However, high R-ISS stage patients or poor treatment responders did not obtain the benefit of this setting, so, another setting with anti-myeloma antibody (i.e. elotuzumab or daratumumab) may be warranted in the future for such patients.
Conclusion: BROAD-J study is conducted to yield the possibility of a careful treatment toward at least PR state and the efficacy of VMP followed by bortezomib maintenance in unfit or frail MM patients. The excellent results we demonstrated such as a good OS, PFS, and a low incidence of AEs might be suggested a superiority of this strategy.
Tokuhira:Bristol Myers Squibb Co., Ltd: Honoraria; Eizai Co., Ltd: Honoraria; Pfizer Co., Ltd: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal